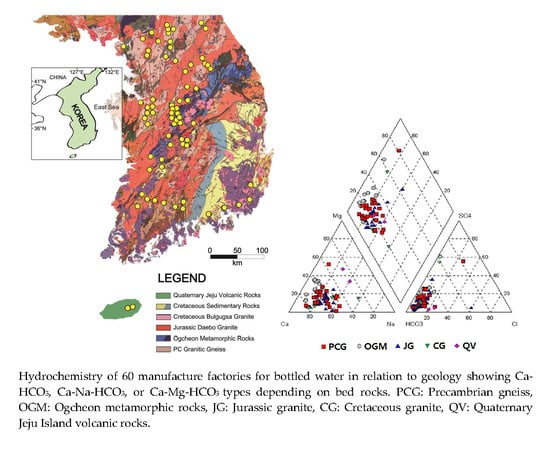
Hydrochemical Properties of Groundwater Used for Korea Bottled Waters in Relation to Geology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geological Classification of Bottled Water Manufactures
2.1.1. Precambrian Gneiss
2.1.2. Ogcheon Metamorphic Rocks
2.1.3. Jurassic Daebo Granite
2.1.4. Cretaceous Bulguksa Granite
2.1.5. Jeju Island Quaternary Volcanic Rocks
2.2. Sampling and Analytical Methods
3. Results and Discussion
3.1. Wells in Manufactures of Bottled Waters
3.2. Hydrochemical Properties of Groundwater
3.2.1. Temperature
3.2.2. pH
3.2.3. EC
3.2.4. Anion Concentrations
3.2.5. Cation Concentrations
3.2.6. Water Types of Groundwater
3.3. Correlation Coefficients
3.4. Groundwater Quality in Relation to Lithology
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reimann, C.; Birke, M. Geochemistry of European Bottled Water; Gebr. Borntraeger Science Publishers: Stuttgart, Germany, 2010. [Google Scholar]
- Ministry of Environment (MOE). Management Legislation on Natural Mineral Water; Ministry of Environment: Sejong, Korea, 2011.
- Kończyk, J.; Muntean, E.; Gega, J.; Frymus, A.; Michalski, R. Major inorganic anions and cations in selected European bottled waters. J. Geochem. Expl. 2019, 197, 27–36. [Google Scholar] [CrossRef]
- Mordor Intelligence. Available online: https://www.mordorintelligence.com/industry-reports/europe-bottled-water-market (accessed on 1 April 2019).
- Ikem, A.; Odueyungbo, S.; Egiebor, N.O.; Nyavor, K. Chemical quality of bottled waters from three cities in eastern Alabama. Sci. Total Env. 2002, 285, 165–175. [Google Scholar] [CrossRef]
- Dinelli, E.; Lima, A.; De Vivo, B.; Albanese, S.; Cicchella, D.; Valera, P. Hydrogeochemical analysis on Italian bottled mineral waters: Effects of geology. J. Geochem. Expl. 2010, 107, 317–335. [Google Scholar] [CrossRef]
- Fugedi, U.; Kuti, L.; Jordan, G.; Kerek, B. Investigation of the hydrogeochemistry of some bottled mineral waters in Hungary. J. Geochem. Expl. 2010, 107, 305–316. [Google Scholar] [CrossRef]
- Lourenço, C.; Ribeiro, L.; Cruz, J. Classification of natural mineral and spring bottled waters of Portugal using principal component analysis. J. Geochem. Expl. 2010, 107, 362–372. [Google Scholar] [CrossRef]
- Bulia, I.L.; Enzweiler, J. The hydrogeochemistry of bottled mineral water in São Paulo state, Brazil. J. Geochem. Expl. 2018, 188, 43–54. [Google Scholar] [CrossRef]
- Brenčič, M.; Ferjan, T.; Gosar, M. Geochemical survey of Slovenian bottled waters. J. Geochem. Expl. 2010, 107, 400–409. [Google Scholar] [CrossRef]
- Lee, B.D. Integrated management system of natural mineral water information. In Proceedings of the Korea Society of Engineering Geology Conference, Gyeongju, Korea, 8–9 April 2010. [Google Scholar]
- Ministry of Environment (MOE). A Study on Characterization Plan of Natural Mineral Water; Ministry of Environment: Sejong, Korea, 2011.
- Ministry of Environment (MOE). Management System of Natural Mineral Water (III); Ministry of Environment: Seoul, Korea, 2000.
- Ministry of Environment (MOE). Regulations and Manual of Drinking Water; Ministry of Environment: Sejong, Korea, 2017.
- Turek, A.; Kim, C.-B. U-Pb zircon ages for Precambrian rocks in southwestern Ryeongnam and southwestern Gyeonggi massifs, Korea. Geochem. J. 1996, 30, 231–249. [Google Scholar] [CrossRef]
- Cho, D.L.; Kwan, S.T. Hornblende geobarometer of the Mesozoic granitoids in South Korea and the evolution of crustal thickness. J. Geol. Soc. Korea 1994, 30, 41–61. [Google Scholar]
- Oh, C.W.; Kim, S.W.; Ryu, I.C.; Okada, T.; Hyodo, H.; Itaya, T. Tectono-metamorphic evolution of the Okcheon metamorphic belt, South Korea: tectonic implications in East Asia. Islan. Arc 2004, 123, 387–402. [Google Scholar] [CrossRef]
- Cho, M.; Cheong, W.; Ernst, W.G.; Yi, K.; Kim, J. SHRIMP U-Pb ages of detrital zircons in metasedimentary rocks of the central Ogcheon fold-thrust belt, Korea: Evidence for tectonic assembly of Paleozoic sedimentary protoliths. J. Asian Ear. Sci. 2013, 63, 234–249. [Google Scholar] [CrossRef]
- Kim, S.W.; Park, S.-I.; Jang, Y.; Kwon, S.; Kim, S.J.; Santosh, M. Tracking Paleozoic evolution of the south Korean peninsula from detrital zircon records: Implications for the tectonic history of East Asia. Gondwana Res. 2017, 50, 195–215. [Google Scholar] [CrossRef]
- Uchida, E.; Choi, S.-G.; Baba, D.; Wakisaka, Y. Petrogenesis and solidification depth of the Jurassic Daebo and Cretaceous Bulguksa granitic rocks in South Korea. Resour. Geol. 2012, 62, 281–295. [Google Scholar] [CrossRef]
- Jwa, Y.J. Possible source rocks of Mesozoic granites in South Korea: Implications for crustal evolution in NE Asia. Trans. R. Soc. Edinb. Earth Sci. 2004, 95, 181–198. [Google Scholar]
- Tsusue, A.; Mizuta, T.; Watanabe, M.; Min, K.G. Jurassic and Cretaceous granitic rocks in South Korea. Min. Geol. 1981, 31, 261–280. [Google Scholar]
- Brenna, M.; Cronin, S.J.; Smith, I.E.M.; Sohn, Y.K. Spatio-temporal evolution of a dispersed magmatic system and its implications for volcano growth, Jeju Island Volcanic Field, Korea. Lithos 2012, 148, 337–352. [Google Scholar] [CrossRef]
- Sohn, Y.K.; Park, K.H. Early-stage volcanism and sedimentation of Jeju Island revealed by the Sagye borehole, SW Jeju Island, Korea. Geosci. J. 2004, 8, 73–84. [Google Scholar] [CrossRef]
- Won, J.H.; Lee, J.Y.; Kim, J.-W.; Koh, G.-W. Groundwater occurrence on Jeju Island, Korea. Hydrogeol. J. 2006, 14, 532–547. [Google Scholar] [CrossRef]
- Won, J.H.; Kim, J.W.; Koh, G.W.; Lee, J.Y. Evaluation of hydrogeological characteristics in Jeju Island, Korea. Geosci. J. 2005, 9, 33–46. [Google Scholar] [CrossRef]
- Drever, J.I. The Geochemisty of Natural Waters, 2nd ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1988. [Google Scholar]
- Ministry of Environment (MOE). Standards for Drinking Water Quality. Ministry of Environment of Korea. Legislation 792 (acted on 1 January 2019). Available online: http://www.law.go.kr/%20lsInfoP.do?%20urlMode=lsInfoP&lsId=007134#0000. (accessed on 20 April 2019).
- Akale, A.T.; Moges, M.A.; Dagnew, D.C.; Tilahun, S.A.; Steenhuis, T.S. Assessment of nitrate in wells and springs in the north central Ethiopian highlands. Water 2018, 10, 476. [Google Scholar] [CrossRef]
- Choo, C.O.; Lee, B.D.; Cho, B.W.; Sung, I.H.; Chi, S.J. Nitrate contamination of confined groundwaters: Application of nitrogen, oxygen, and hydrogen isotopes. J. Eng. Geol. 2002, 12, 285–294. [Google Scholar]
- White, A.F.; Brantley, S.L. Chemical weathering rates of silicate minerals: An overview. In Chemical Weathering Rates of Silicate Minerals; White, A.F., Brantley, S.L., Eds.; Mineralogical Society of America: Washington, DC, USA, 1995; Reviews in Mineralogy; Volume 31, pp. 1–22. [Google Scholar]
- Fournier, R.O.; Rowe, J.J. The solubility of amorphous silica in water at high temperatures and high pressures. Am. Miner. 1977, 62, 1052–1056. [Google Scholar]
- Rimstidt, J.D.; Barnes, H.L. The kinetics of silica-water reactions. Geochim. Cosmochim. Acta 1980, 44, 1683–1699. [Google Scholar] [CrossRef]
- Rimstidt, J.D. Quartz solubility at low temperatures. Geochim. Cosmochim. Acta 1997, 61, 2553–2558. [Google Scholar] [CrossRef]
- Lasaga, A.C. Chemical kinetics of water-rock interactions. J. Geophy. Res. 1984, 89, 4009–4025. [Google Scholar] [CrossRef]
- Chou, L.; Wollast, R. Steady-state kinetics and dissolution mechanisms of albite. Am. J. Sci. 1985, 285, 963–993. [Google Scholar] [CrossRef]
- Chebotarev, I.I. Metamorphism of natural waters in the crust of weathering-1. Geochim. Cosmochim. Acta 1955, 8, 22–48. [Google Scholar] [CrossRef]
- Freeze, R.A.; Cherry, J.A. Groundwater; Prentice Hall Inc.: Englewood Cliffs, NJ, USA, 1979. [Google Scholar]
- Frengstad, B.; Banks, D.; Skrede, A.M.; Krog, J.R.; Siewers, U.; Strand, T. The hydrochemistry of crystalline bedrock groundwater in Norway. NGU Bull. 2002, 439, 87–98. [Google Scholar]
- Demetriades, A.; Reimann, C.; Birke, M. The geological atlas of European ground water with emphasis on Hellas. Bull. Geol. Soc. Greece 2012, 46, 39–80. [Google Scholar] [CrossRef]
- Ishaku, J.M. Hydrochemical Evolution of Groundwater in Jimeta-Yola Area, Northeastern Nigeria. Global J. Geol. Sci. 2011, 9, 99–121. [Google Scholar]
- Ravikumar, P.; Somashekar, R.K. Principal component analysis and hydrochemical facies characterization to evaluate groundwater quality in Varahi river basin, Karnataka state, India. Appl. Water Sci. 2017, 7, 745–755. [Google Scholar] [CrossRef]
- Ray, R.K.; Mukherjee, R. Hydrochemical evolution of groundwater in the phreatic aquifers of Chhattisgarh. J. Geol. Soc. India 2008, 72, 405–414. [Google Scholar]
- Saravanan, K.; Srinivasamoorthy, K.; Prakash, R.; Gopinath, S.; Suma, C.S. An evaluation of hydrogeochemistry of groundwater in upper Vellar sub–basin using mineral stability and solute transport modelling. Aqua. Procedia 2015, 4, 1119–1125. [Google Scholar] [CrossRef]
- Oyebog, S.A.; Ako, A.A.; Nkeng, G.E.; Suh, E.C. Hydrogeochemical characteristics of some Cameroon bottled waters, investigated by multivariate statistical analyses. J. Geochem. Expl. 2012, 112, 118–130. [Google Scholar] [CrossRef]
- Misund, A.; Frengstad, B.; Sewersd, U.; Reimann, C. Variation of 66 elements in European bottled mineral waters. Sci. Total Envion. 1999, 243–244, 21–41. [Google Scholar] [CrossRef]



| Samples | K | Na | Ca | Mg | SiO2 | F | Cl | SO4 | NO3-N | HCO3 | EC | pH | T (°C) | Depth (m) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCG1 | 2.36 | 2.66 | 8.35 | 1.68 | 8.88 | 0.06 | 4.98 | 7.83 | 4.92 | 12.20 | 102.0 | 7.30 | 15.9 | 198.0 |
| PCG2 | 0.69 | 2.52 | 5.79 | 0.65 | 9.86 | 0.04 | 2.56 | 4.10 | 1.12 | 24.40 | 81.5 | 8.45 | 18.3 | 264.4 |
| PCG3 | 0.55 | 1.69 | 4.22 | 0.90 | 7.47 | 0.04 | 1.57 | 3.82 | 1.67 | 24.40 | 48.4 | 7.28 | 13.4 | 178.3 |
| PCG4 | 1.34 | 6.39 | 31.30 | 1.56 | 11.10 | 0.27 | 5.35 | 13.10 | 1.63 | 106.75 | 222.5 | 7.24 | 12.3 | 221.3 |
| PCG5 | 0.89 | 4.12 | 6.36 | 0.97 | 17.30 | 0.10 | 2.46 | 4.80 | 2.50 | 27.45 | 65.5 | 5.98 | 11.1 | 146.6 |
| PCG6 | 1.49 | 8.80 | 12.50 | 2.10 | 12.40 | 0.60 | 2.52 | 7.98 | 1.22 | 79.30 | 140.5 | 7.27 | 13.2 | 166.7 |
| PCG7 | 1.44 | 8.04 | 22.20 | 19.30 | 8.94 | 0.14 | 6.38 | 15.30 | 0.85 | 173.85 | 344.0 | 7.50 | 11.7 | 126.2 |
| PCG8 | 0.61 | 2.75 | 7.61 | 1.69 | 17.30 | 0.07 | 1.91 | 1.96 | 0.56 | 39.65 | 76.4 | 7.36 | 14.1 | 186.8 |
| PCG9 | 0.86 | 3.97 | 10.40 | 0.87 | 19.60 | 0.18 | 2.12 | 2.06 | 0.30 | 42.70 | 90.5 | 7.01 | 17.5 | 310.0 |
| PCG10 | 0.90 | 4.45 | 11.70 | 1.10 | 20.20 | 0.15 | 2.78 | 3.13 | 1.68 | 42.70 | 102.2 | 7.22 | 16.8 | 197.0 |
| PCG11 | 0.76 | 3.68 | 6.53 | 0.86 | 25.70 | 0.08 | 1.78 | 2.03 | 0.65 | 27.45 | 68.3 | 6.27 | 15.7 | 101.4 |
| PCG12 | 0.56 | 6.13 | 5.42 | 0.43 | 11.40 | 0.27 | 1.50 | 7.17 | 0.28 | 27.45 | 66.4 | 6.97 | 13.5 | 184.5 |
| PCG13 | 2.25 | 22.50 | 34.30 | 3.55 | 23.90 | 1.32 | 12.40 | 12.00 | 0.02 | 146.40 | 328.0 | 7.15 | 17.2 | 110.7 |
| PCG14 | 0.42 | 18.10 | 17.60 | 0.96 | 21.20 | 0.24 | 4.33 | 12.30 | 1.11 | 70.15 | 196.0 | 7.90 | 16.4 | 323.5 |
| PCG15 | 2.06 | 6.03 | 19.00 | 1.70 | 19.90 | 0.15 | 3.43 | 11.00 | 0.25 | 76.25 | 157.0 | 7.57 | 16.0 | 242.5 |
| PCG16 | 0.25 | 17.70 | 17.80 | 0.24 | 29.70 | 1.09 | 6.73 | 13.50 | 0.43 | 85.40 | 199.0 | 8.10 | 20.9 | 400.0 |
| PCG17 | 1.22 | 17.50 | 38.20 | 3.27 | 18.30 | 0.64 | 17.90 | 30.00 | 0.28 | 112.85 | 331.0 | 6.58 | 15.8 | 219.0 |
| PCG18 | 1.36 | 8.34 | 44.70 | 9.68 | 21.80 | 0.26 | 12.80 | 27.60 | 0.73 | 161.65 | 309.0 | 6.60 | 17.1 | 187.0 |
| PCG19 | 1.13 | 10.80 | 21.00 | 5.28 | 35.90 | 1.13 | 10.90 | 6.78 | 0.37 | 94.55 | 201.0 | 7.21 | 17.6 | 197.0 |
| PCG20 | 0.96 | 7.08 | 19.40 | 1.82 | 16.50 | 0.09 | 3.85 | 14.70 | 0.22 | 64.05 | 149.0 | 7.19 | 14.0 | 200.0 |
| PCG21 | 2.26 | 11.90 | 24.10 | 9.71 | 38.30 | 0.18 | 5.20 | 3.97 | 0.23 | 167.75 | 266.7 | 6.37 | 11.4 | 222.9 |
| PCG22 | 1.81 | 9.78 | 22.00 | 3.98 | 30.40 | 0.58 | 3.89 | 7.28 | 0.07 | 103.70 | 236.7 | 7.21 | 16.3 | 188.1 |
| PCG23 | 0.74 | 5.56 | 16.80 | 2.33 | 13.90 | 0.06 | 7.17 | 11.50 | 0.99 | 53.38 | 136.0 | 6.33 | 14.2 | 178.5 |
| PCG24 | 0.83 | 9.94 | 31.40 | 4.24 | 18.80 | 0.08 | 11.50 | 17.40 | 0.12 | 114.38 | 240.0 | 7.52 | 15.0 | 204.8 |
| PCG25 | 1.47 | 13.50 | 28.00 | 5.55 | 45.60 | 0.24 | 9.87 | 11.30 | 2.73 | 118.95 | 254.0 | 6.62 | 15.1 | 141.6 |
| PCG26 | 2.34 | 10.90 | 22.30 | 5.84 | 35.50 | 0.39 | 5.98 | 6.56 | 1.30 | 118.95 | 211.0 | 5.97 | 14.9 | 203.7 |
| OGM1 | 0.77 | 6.66 | 9.74 | 1.63 | 30.20 | 0.32 | 4.43 | 2.77 | 0.79 | 54.90 | 107.9 | 7.83 | 16.0 | 210.0 |
| OGM2 | 2.02 | 11.30 | 31.10 | 6.90 | 34.00 | 0.33 | 5.09 | 9.88 | 0.41 | 183.00 | 283.8 | 7.35 | 14.3 | 141.0 |
| OGM3 | 1.53 | 18.30 | 25.70 | 13.20 | 15.80 | 0.76 | 3.59 | 25.50 | 0.01 | 167.75 | 333.0 | 8.34 | 15.0 | 143.0 |
| OGM4 | 1.90 | 6.20 | 49.30 | 7.19 | 37.70 | 0.83 | 3.60 | 72.80 | 0.63 | 103.70 | 340.0 | 7.13 | 16.3 | 117.2 |
| OGM5 | 1.17 | 6.18 | 17.80 | 4.69 | 18.80 | 0.06 | 5.91 | 7.07 | 3.56 | 57.95 | 162.3 | 6.31 | 14.1 | 159.3 |
| OGM6 | 0.70 | 6.56 | 11.90 | 0.98 | 16.30 | 0.29 | 2.31 | 3.35 | 0.19 | 57.95 | 95.4 | 8.30 | 13.5 | 287.5 |
| OGM7 | 1.08 | 2.59 | 17.60 | 1.82 | 11.20 | 0.06 | 5.91 | 7.07 | 2.37 | 54.90 | 123.2 | 7.05 | 12.2 | 182.7 |
| OGM8 | 0.73 | 1.96 | 13.40 | 5.22 | 11.10 | 1.69 | 7.58 | 0.07 | 0.00 | 67.10 | 121.0 | 7.74 | 13.5 | 139.0 |
| OGM9 | 0.84 | 4.39 | 32.00 | 3.19 | 11.90 | 0.06 | 2.48 | 26.50 | 0.00 | 106.75 | 227.0 | 8.30 | 14.1 | 188.1 |
| OGM10 | 1.55 | 9.07 | 33.50 | 8.18 | 24.80 | 0.66 | 7.73 | 18.20 | 0.91 | 143.35 | 284.0 | 7.69 | 16.3 | 204.8 |
| OGM11 | 0.70 | 6.90 | 29.80 | 11.30 | 13.20 | 0.27 | 4.16 | 24.20 | 0.70 | 152.50 | 272.0 | 7.41 | 14.5 | 113.1 |
| JG1 | 0.80 | 8.78 | 15.70 | 2.95 | 35.30 | 0.34 | 3.88 | 9.93 | 1.99 | 73.20 | 167.3 | 6.78 | 14.2 | 103.2 |
| JG2 | 0.38 | 17.60 | 18.90 | 0.36 | 23.70 | 1.90 | 33.20 | 4.48 | 0.06 | 48.80 | 204.0 | 7.48 | 18.6 | 110.0 |
| JG3 | 0.56 | 5.02 | 18.60 | 1.01 | 14.70 | 0.55 | 4.56 | 4.11 | 0.85 | 79.30 | 136.3 | 6.95 | 11.0 | 244.0 |
| JG4 | 0.61 | 13.00 | 20.60 | 1.72 | 24.80 | 1.60 | 4.58 | 5.46 | 0.93 | 97.60 | 195.7 | 6.80 | 10.2 | 317.8 |
| JG5 | 0.63 | 8.27 | 15.10 | 2.39 | 8.79 | 0.25 | 2.59 | 8.37 | 2.03 | 76.25 | 161.8 | 7.36 | 12.6 | 190.0 |
| JG6 | 0.48 | 23.60 | 17.70 | 5.90 | 12.00 | 0.91 | 5.28 | 15.40 | 2.82 | 128.10 | 245.0 | 8.12 | 14.5 | 218.0 |
| JG7 | 0.39 | 6.85 | 14.80 | 1.27 | 20.70 | 0.83 | 1.39 | 2.99 | 0.39 | 76.25 | 111.1 | 7.08 | 12.9 | 205.0 |
| JG8 | 0.59 | 4.97 | 10.20 | 1.56 | 23.50 | 0.16 | 1.90 | 0.63 | 1.37 | 51.85 | 94.2 | 7.47 | 10.6 | 205.2 |
| JG9 | 0.97 | 12.20 | 16.50 | 1.99 | 23.10 | 0.91 | 4.06 | 9.47 | 2.28 | 82.35 | 116.3 | 6.65 | 14.5 | 154.2 |
| JG10 | 0.52 | 17.70 | 41.60 | 1.77 | 25.70 | 0.17 | 13.10 | 7.77 | 1.76 | 189.10 | 37.5 | 7.36 | 15.7 | 243.0 |
| JG11 | 0.52 | 5.49 | 10.40 | 1.41 | 26.30 | 0.17 | 1.68 | 1.04 | 0.76 | 61.00 | 98.2 | 6.44 | 14.4 | 172.9 |
| JG12 | 0.80 | 4.45 | 51.90 | 1.08 | 27.20 | 0.07 | 1.54 | 1.48 | 0.34 | 45.75 | 64.1 | 6.19 | 14.7 | 229.3 |
| JG13 | 1.12 | 4.45 | 10.40 | 0.88 | 19.70 | 0.25 | 1.22 | 2.68 | 0.58 | 54.90 | 89.3 | 7.18 | 13.2 | 141.1 |
| JG14 | 0.78 | 6.62 | 15.20 | 1.45 | 19.40 | 0.12 | 5.66 | 6.85 | 2.64 | 57.95 | 122.2 | 6.50 | 13.2 | 140.0 |
| JG15 | 0.42 | 5.36 | 12.60 | 0.88 | 21.20 | 0.95 | 3.12 | 5.66 | 0.98 | 45.75 | 104.1 | 6.94 | 13.3 | 187.5 |
| JG16 | 0.59 | 12.00 | 13.30 | 1.58 | 34.70 | 0.42 | 7.06 | 7.16 | 4.59 | 48.80 | 153.5 | 5.97 | 15.7 | 100.0 |
| CG1 | 0.42 | 10.50 | 3.84 | 1.36 | 54.60 | 0.11 | 3.62 | 3.35 | 0.07 | 48.80 | 85.8 | 7.02 | 18.6 | 197.0 |
| CG2 | 0.10 | 5.22 | 25.60 | 0.71 | 27.20 | 0.07 | 2.32 | 43.80 | 0.04 | 42.70 | 187.5 | 7.81 | 17.8 | 294.5 |
| CG3 | 0.28 | 14.20 | 16.90 | 3.43 | 43.00 | 0.41 | 3.20 | 3.49 | 0.15 | 111.33 | 168.8 | 7.02 | 19.8 | 196.2 |
| CG4 | 0.87 | 11.40 | 36.30 | 1.89 | 29.70 | 0.29 | 4.00 | 9.23 | 0.41 | 146.40 | 258.1 | 7.01 | 16.5 | 216.5 |
| CG5 | 0.44 | 6.86 | 10.20 | 1.78 | 35.90 | 0.24 | 3.52 | 2.80 | 0.34 | 51.85 | 102.0 | 7.14 | 16.0 | 177.7 |
| QV1 | 2.11 | 5.35 | 2.81 | 2.23 | 28.20 | 0.05 | 5.93 | 1.45 | 0.10 | 70.15 | 68.0 | 7.96 | 14.0 | 420.0 |
| QV2 | 2.56 | 7.47 | 6.35 | 6.58 | 38.20 | 0.03 | 8.54 | 1.71 | 0.61 | 70.15 | 130.4 | 7.43 | 15.9 | 325.0 |
| PCG | K | Na | Ca | Mg | SiO2 | F | Cl | SO4 | NO3 | HCO3 | EC | pH |
| K | 1.00 | |||||||||||
| Na | 0.20 | 1.00 | ||||||||||
| Ca | 0.38 | 0.63 | 1.00 | |||||||||
| Mg | 0.40 | 0.18 | 0.47 | 1.00 | ||||||||
| SiO2 | 0.30 | 0.50 | 0.35 | 0.18 | 1.00 | |||||||
| F | 0.18 | 0.73 | 0.38 | 0.01 | 0.39 | 1.00 | ||||||
| Cl | 0.24 | 0.65 | 0.85 | 0.36 | 0.30 | 0.49 | 1.00 | |||||
| SO4 | 0.06 | 0.49 | 0.81 | 0.33 | −0.05 | 0.21 | 0.80 | 1.00 | ||||
| NO3 | 0.19 | −0.32 | −0.28 | −0.12 | −0.17 | −0.33 | −0.17 | −0.18 | 1.00 | |||
| HCO3 | 0.49 | 0.62 | 0.84 | 0.78 | 0.44 | 0.38 | 0.64 | 0.55 | 0.35 | 1.00 | ||
| EC | 0.45 | 0.73 | 0.90 | 0.69 | 0.37 | 0.46 | 0.79 | 0.71 | −0.29 | 0.94 | 1.00 | |
| pH | −0.30 | 0.05 | −0.16 | −0.16 | −0.34 | 0.12 | −0.15 | −0.01 | −0.10 | −0.14 | −0.06 | 1.00 |
| OGM | K | Na | Ca | Mg | SiO2 | F | Cl | SO4 | NO3 | HCO3 | EC | pH |
| K | 1.00 | |||||||||||
| Na | 0.53 | 1.00 | ||||||||||
| Ca | 0.68 | 0.23 | 1.00 | |||||||||
| Mg | 0.42 | 0.69 | 0.52 | 1.00 | ||||||||
| SiO2 | 0.70 | 0.26 | 0.47 | 0.05 | 1.00 | |||||||
| F | 0.08 | 0.00 | 0.04 | 0.30 | 0.02 | 1.00 | ||||||
| Cl | 0.12 | 0.65 | −0.15 | 0.08 | −0.03 | 0.43 | 1.00 | |||||
| SO4 | 0.51 | 0.15 | 0.89 | 0.41 | 0.44 | 0.10 | −0.35 | 1.00 | ||||
| NO3 | 0.20 | −0.26 | −0.20 | −0.24 | −0.05 | −0.47 | 0.36 | −0.19 | 1.00 | |||
| HCO3 | 0.58 | 0.72 | 0.61 | 0.81 | 0.24 | 0.06 | −0.06 | 0.32 | −0.42 | 1.00 | ||
| EC | 0.73 | 0.73 | 0.88 | 0.82 | 0.40 | 0.11 | −0.13 | 0.74 | −0.27 | 0.85 | 1.00 | |
| pH | −0.27 | 0.05 | −0.12 | 0.04 | −0.23 | 0.20 | −0.45 | −0.07 | −0.85 | 0.20 | 0.02 | 1.00 |
| JG | K | Na | Ca | Mg | SiO2 | F | Cl | SO4 | NO3 | HCO3 | EC | pH |
| K | 1.00 | |||||||||||
| Na | −0.32 | 1.00 | ||||||||||
| Ca | 0.03 | 0.16 | 1.00 | |||||||||
| Mg | −0.04 | 0.59 | −0.05 | 1.00 | ||||||||
| SiO2 | 0.11 | −0.07 | 0.16 | −0.25 | 1.00 | |||||||
| F | −0.39 | 0.50 | −0.15 | −0.03 | −0.07 | 1.00 | ||||||
| Cl | −0.37 | 0.56 | 0.14 | −0.21 | 0.10 | 0.58 | 1.00 | |||||
| SO4 | 0.00 | 0.71 | −0.04 | 0.81 | −0.19 | 0.18 | 0.09 | 1.00 | ||||
| NO3 | 0.11 | 0.34 | −0.19 | 0.49 | 0.16 | −0.25 | −0.13 | 0.63 | 1.00 | |||
| HCO3 | −0.18 | 0.64 | 0.38 | 0.47 | −0.16 | 0.00 | 0.10 | 0.51 | 0.16 | 1.00 | ||
| EC | −0.26 | 0.56 | −0.37 | 0.54 | −0.22 | 0.62 | 0.31 | 0.57 | 0.25 | −0.03 | 1.00 | |
| pH | −0.30 | 0.49 | −0.14 | 0.46 | −0.61 | 0.28 | 0.27 | 0.34 | −0.19 | 0.45 | 0.35 | 1.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.D.; Oh, Y.H.; Cho, B.W.; Yun, U.; Choo, C.O. Hydrochemical Properties of Groundwater Used for Korea Bottled Waters in Relation to Geology. Water 2019, 11, 1043. https://doi.org/10.3390/w11051043
Lee BD, Oh YH, Cho BW, Yun U, Choo CO. Hydrochemical Properties of Groundwater Used for Korea Bottled Waters in Relation to Geology. Water. 2019; 11(5):1043. https://doi.org/10.3390/w11051043
Chicago/Turabian StyleLee, Byeong Dae, Yong Hwa Oh, Byong Wook Cho, Uk Yun, and Chang Oh Choo. 2019. "Hydrochemical Properties of Groundwater Used for Korea Bottled Waters in Relation to Geology" Water 11, no. 5: 1043. https://doi.org/10.3390/w11051043