Factsheet about West Nile virus infection
West Nile virus (WNV) infection is a mosquito-borne zoonosis that is endemo-epidemic in Europe. The disease affects countries in southern, eastern and western Europe [1–3]. The virus is transmitted among birds via the bite of infected mosquitoes and incidentally humans and other mammals may become infected. About 80% of WNV infections in humans are asymptomatic [4]. West Nile fever (WNF) is the most common clinical presentation. The elderly and immunocompromised persons are at higher risk of developing West Nile neuroinvasive disease (WNND). No specific prophylaxis or treatment exist against the disease in humans.
Case definition
Cases of WNV infection should be notified to the European Centre for Disease Prevention and Control (ECDC) following the EU case definition outlined in Decision (EU) 2018/945/2018/EC [5].
Clinical criteria
At least one of the following:
- any person with fever
- encephalitis; and/or
- meningitis
Laboratory criteria
Laboratory test for case confirmation
At least one of the following:
- isolation of WNV from blood or cerebrospinal fluid (CSF)
- detection of WNV nucleic acid in blood or CSF
- WNV-specific antibody response (immunoglobulin M; IgM) in CSF; and/or
- WNV IgM high titre, detection of WNV IgG and confirmation by neutralisation
Laboratory test for probable case
WNV-specific antibody response in serum.
Epidemiological criteria
At least one of the following epidemiological links:
- Animal to human transmission (residing, having visited or having been exposed to mosquito bites in an area where WNV is endemic in horses or birds)
- Human to human transmission (vertical transmission, blood transfusion, transplants)
Case classification
A. Possible case
Not applicable
B. Probable case
Any person meeting the clinical criteria and with at least:
- an epidemiological link; and
- a laboratory test for a probable case.
C. Confirmed case
Any person meeting laboratory criteria for case confirmation
Note: Serological results should be interpreted according to previous exposure to other flavivirus infections and vaccination status. Confirmed cases in such situations should be validated by serum neutralisation or other equivalent assays.
The pathogen
WNV is an enveloped positive-stranded ribonucleic acid (RNA) virus belonging to the Japanese encephalitis serocomplex (Flavivirus genus, Flaviviridae family) [6]. Eight phylogenetic lineages have been described [7], but only lineage 1 and 2 are associated with disease in humans [8].
WNV was first isolated in 1937 from a woman in the West Nile district in Uganda. It is currently the arbovirus with the widest geographic distribution and can be found in parts of North and South America, Africa, Europe, Asia and Oceania.
Clinical features and sequelae
Humans
Most WNV infections in humans are asymptomatic. About 20% of WNV infections in humans may cause WNF and less than one percent may cause WNND [4]. Very rarely, WNV infection leads to Guillain–Barré syndrome and other demyelinating neuropathies [9].
WNF is characterised by a sudden onset of symptoms that may include headache, malaise, fever, myalgia, vomiting, rash, fatigue and eye pain [9]. Symptom severity ranges from a mild self-limiting illness from which patients recover within one week to a protracted debilitating disease that can last for months [10,11].
WNND involves symptoms that affect the central nervous system. These can be categorised clinically as meningitis, encephalitis and acute flaccid paralysis or a combination of the three. Risk factors include advanced age, malignancies disrupting the blood–brain barrier, hypertension, hematologic disorders, diabetes mellitus, renal disease, alcohol abuse and genetic factors [9,12]. The case fatality ratio among patients with WNND can be up to 17% [13,14].
Animals
WNV infections among equids are usually asymptomatic. Approximately 10% may show neurological signs that can range from mild ataxia to total recumbence [28].
Certain bird species, especially raptors and corvids, tend to be more sensitive to WNV infection and frequently develop clinical signs that lead to fatal outcomes. Avian mass mortality events are influenced by geographic and environmental factors, as well as by genetic differences of the WNV strains [15].
Transmission
WNV is transmitted in an enzootic cycle between mosquitoes and birds that respective acting as vectors and amplifying hosts. Mammals can become infected from the bite of an infected mosquito, but are considered dead-end hosts.
Figure 1. West Nile virus transmission cycle
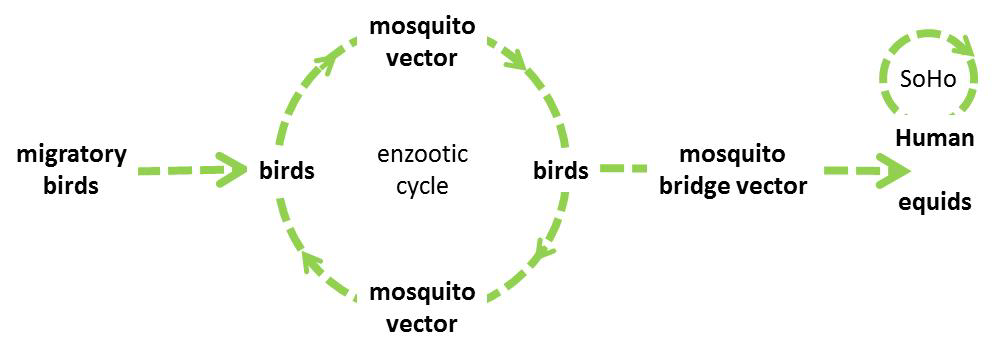
WNV is introduced in Europe through migratory birds travelling from sub-Saharan Africa, North Africa or the Middle East and overwinters in mosquitoes [16], while overwintering in local bird species in Europe cannot be excluded.
In Europe, the mosquito species Culex pipiens and Culex modestus are the main vectors of WNV. Aedes albopictus and Aedes (Ochlerotatus) detritus are also competent vectors in laboratory setting but are considered to be of little importance for transmission as these species mainly bite mammals and are thus unlikely to acquire the infection from birds [17]. Culex pipiens encompasses two behaviourally distinct biotypes, Cx. pipiens pipiens and Cx. pipiens molestus that can form hybrids in peri-urban areas [18,19]. The Cx. pipiens pipiens biotype prefers feeding on birds and plays an important role in the enzootic transmission cycle of WNV. The Cx. pipiens molestus biotype feeds mostly on mammals and probably plays a limited role in transmission. However, hybrids feed on both birds and mammals and likely play an important role as a bridge vector in the epizootic transmission of the virus to humans and equids [20]. Cx. pipiens overwinters in adult form.
Transmission of WNV occurs when mosquitoes are active (i.e. between spring and autumn) and most infections in humans and equids are observed between July and September [2,3].
In humans, the incubation period is usually two to six days, although incubation periods of up to 21 days have been reported in immunocompromised people [9]. The viremia in humans is low, occurring one to three days after infection, can last up to 11 days [21] and is not considered to be infectious to mosquitoes.
However, human-to-human transmission may occur through substances of human origin (SoHO): transfusion of blood and blood components or transplantation of tissues, cells or organs from an infected and viraemic donor [22]. In the United States, one case of vertical transplacental mother-to-child transmission and one case of transmission to an infant through breastfeeding were described [23,24]. WNV infections following occupational exposure (i.e. while collecting mosquitoes, performing autopsies or other laboratory work) were also documented [25–27].
Birds are able to shed the virus at high titres through oral and cloacal secretions and direct transmission between certain birds species (e.g. geese) was documented [28,29].
Epidemiology
Serological surveys have demonstrated WNV circulation in Europe since the 1950s [30]. The first recognised outbreak in humans occurred in 1962–1963 in the Camargue, southern France [31]. In 1996, the first major WNV infection epidemic occurred in Europe, during which Romania identified about 400 cases [32]. Since then, cases and outbreaks have been reported in southern, eastern and western European countries [1–3].
Temperature has been cited as one of the important environmental variables modulating WNV activity in Europe as it affects both mosquito breeding and the extrinsic incubation of WNV [33]. Above-normal summer temperatures are considered among of the factors directly influencing dispersal into new areas and amplification [8]. For Europe, a study showed that temperature anomalies in July, sufficient water surfaces in June, the presence of wetlands and locations under migratory bird routes, as well as the presence of WNV in previous years, are associated with new cases [34].
Prior to the emergence of a lineage 2 strain in Hungary in 2004, sporadic cases and occasional outbreaks in animals and humans in Europe were due to lineage 1 strains [6]. Since 2008, the lineage 2 strain has spread over central Europe and the eastern Mediterranean region. This strain caused significant outbreaks in humans and in animals in several counties, including Greece, Hungary, and Serbia. Another lineage 2 virus emerged in southern Russia in 2007, which spread to Romania and Italy in and after 2010 [35–37]. In certain countries (i.e. Italy, Romania and Turkey), neurovirulent WNV strains that belong to different genetic lineages circulate simultaneously.
Diagnostics
Laboratory methods for the diagnosis of a WNV infection are most commonly indirect detection based on serology, but can also entail direct detection of the virus. The samples of choice are whole blood, plasma, serum, CSF (in case of neurological implications) and urine for genome detection and serum and CSF (in case of neurological implications) for serology.
Indirect detection
Indirect detection of a WNV infection is based on detection of WNV-specific IgM and/or IgG. Multiple in-house and commercial serologic tests exist mainly based on enzyme-linked immunosorbent assay (ELISA) or indirect immunofluorescence (IIFA) principles.
WNV infection is mostly determined by detection of WNV-specific IgM in serum and/or CSF. Seroconversion for IgM typically occurs three to eight days post onset of WNF symptoms and IgM usually persists for 30–90 days. However, longer persistence has been described with the presence of IgM up to three years post-onset of symptoms. This implies that a negative WNV IgM result in serum drawn less than eight days post-onset of symptoms does not exclude a WNV infection, while the presence of WNV-specific IgM does not always indicate a recent infection. IgM detection in CSF of a patient with a functional blood–brain barrier points at an infection of the central nervous system. In patients with neuro-invasive disease, IgM can be detected in CSF usually one to eight days after onset of neurological symptoms.
WNV-specific IgG is typically detectable from eight days post onset of symptoms onwards and may persist for multiple years. Therefore, diagnosis based on IgG requires testing of an acute and convalescent serum to demonstrate seroconversion or a minimal four-fold increase in IgG titers.
WNV diagnosis based on serology is severely hampered by extensive cross-reactivity between antibodies triggered by related viruses of the genus Flavivirus (i.e. endemic flaviviruses such as tick-borne encephalitis and Usutu virus and exotic or travel-associated flavivirus infections such as dengue and Zika virus) or by vaccination (e.g. yellow fever, tick-borne encephalitis, dengue – very limited market – and Japanese encephalitis vaccines). In addition, an acute flavivirus infection may boost cross-reactive antibodies due to previous infection with or vaccination against another flavivirus, thereby obscuring antibody response to the present acute infection. ELISA and IIFA testing are only valuable as screening tests. Positive results obtained with these type of assays should be confirmed by virus neutralisation tests (VNT; plaque reduction neutralisation test – PRNT80 and PRNT90), preferably by parallel testing of acute and convalescent serum samples to distinguish recent infection from past infections. To exclude cross-reactions with other flaviviruses, PRNTs should be performed simultaneously against all potential flaviviruses. Such tests require biosafety level 3 facilities and appropriate reference viruses.
Direct detection
West Nile virus infection can be confirmed by virus genome detection or virus isolation. Viral genome is typically detectable in plasma from 2–18 days post-infection and up to five days post-onset of symptoms, although prolonged viremia (up to 35 days after symptom onset) has been reported. There is evidence that whole blood is the best sample type, with 86.8% sensitivity, while sensitivity is lower in urine, plasma, serum and CSF [38]. Requirements for sensitivity of assays used depend on setting, e.g. SoHO screening of population samples versus identification of clinical cases.
Virus isolation is not considered as a test of choice for diagnosis, as it requires biosafety level 3 facilities and it takes up to five days to obtain a cytopathogenic effect. WNV antigen detection by immunohistochemistry is possible in post-mortem tissue of fatal encephalitis cases.
Case management and treatment
No specific prophylaxis or treatment exist for WNV infections. The only available treatment is supportive care [9].
Public health control measures
Measures to prevent WNV transmission via SoHO
To prevent transfusion-transmitted WNV infections, EU/EEA countries should implement 28-day blood donor deferral or individual donation nucleic acid testing (ID-NAT) of prospective donors who have visited or live in an affected area. In affected areas, blood establishments [1] should follow recommendations provided in the EU preparedness plan for blood safety [39]. Donors of organs, tissues and cells living in or returning from an affected area should be tested for WNV infection. Systematic collection of epidemiological information on WNV infection among donors and recipients of SoHO is an important tool for national authorities to better assess the risk of transmission and impact of preventive measures on the availability of SoHO. According to the preparedness plan for WNV blood safety in the EU [39], blood establishments in affected areas should:
- temporarily interrupt blood collection or implement NAT screening for blood donations from WNV affected areas
- quarantine, retest and discard positive blood components in storage at the time of implementation of measures and retrieve and quarantine blood components derived from whole blood donated 120 days prior the date of collection of the ID-NAT-positive donation
- enhance donor post-donation information, especially about fever, influenza-like illness or other acute symptoms within 15 days after donation
- strengthen post-transfusion haemovigilance and perform look-back analysis in any case of transfusion-transmitted WNV infection for a period dating 120 days prior to the donation of implicated blood components; and
- consider the use of pathogen inactivation procedures.
[1] According to Directive 2002/98/EC, ‘blood establishments’ are structures or bodies that are responsible for any aspect of the collection and testing of human blood or blood components, whatever their intended purpose, and their processing, storage, and distribution when intended for transfusion.
Vector control
Mosquito vectors may be controlled through larval source reduction and measures against adult mosquitoes. Vector breeding sites include stagnant and often dirty water collections in dishes, buckets, barrels and cans, flowerpots, rain gutters, discarded tires and other containers that can collect water. In urban environments, infrastructure such as underground heating, sewage pipes and basements liable to flooding can act as breeding and resting sites for vectors.
Specific methods for vector control to prevent transmission of WNV have been infrequently evaluated for their impact on reducing human cases [40].
Infection control, personal protection and prevention
Personal protective measures against mosquito bites include the use of mosquito bed nets (preferably insecticide-treated nets), sleeping or resting in screened or air-conditioned rooms, the wearing of clothes that cover most of the body, and the use of mosquito repellent in accordance with the instructions indicated on the product label.
Read more on the ECDC website
Read more on external websites
References
- United Nations Statistics Division. Standard Country or Area Codes for Statistical Use. New York: U N; 2018 [cited 20 September 2018]. Available from: https://unstats.un.org/unsd/methodology/m49.
- European Centre for Disease Prevention and Control. West Nile fever. In: ECDC. Annual epidemiological report for 2015. Stockholm: ECDC; 2017 [cited 20 September 2018]. Available from: http://ecdc.europa.eu/publications-data/west-nile-fever-annual-epidemio….
- European Center for Disease Prevention and Control. Weekly updates: 2018 West Nile fever transmission season. Stockholm: ECDC; 2018 [cited 20 September 2018]. Available from: http://ecdc.europa.eu/west-nile-fever/surveillance-and-disease-data/dis….
- Sambri V, Capobianchi M, Charrel R, Fyodorova M, Gaibani P, Gould E, et al. West Nile virus in Europe: emergence, epidemiology, diagnosis, treatment, and prevention. Clin Microbiol Infect. 2013 Aug;19(8):699-704.
- European Commission. Commission implementing decision 2018/945 of 22 June 2018 on the communicable diseases and related special health issues to be covered by epidemiological surveillance as well as relevant case definitions. (Text with EEA relevance). Luxembourg: European Commission; 2018. Available from: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018D0945….
- Zeller H, Schuffenecker I. West Nile virus: an overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. Eur J Clin Microbiol Infect Dis. 2004 Mar;23(3):147-56.
- Fall G, Di Paola N, Faye M, Dia M, Freire CCM, Loucoubar C, et al. Biological and phylogenetic characteristics of West African lineages of West Nile virus. PLoS Negl Trop Dis. 2017 Nov;11(11):e0006078.
- Paz S. Climate change impacts on West Nile virus transmission in a global context. Philos Trans R Soc Lond B Biol Sci. 2015 Apr 5;370(1665).
- Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013 Jul 17;310(3):308-15.
- Sejvar JJ, Curns AT, Welburg L, Jones JF, Lundgren LM, Capuron L, et al. Neurocognitive and functional outcomes in persons recovering from West Nile virus illness. J Neuropsychol. 2008 Sep;2(Pt 2):477-99.
- Sejvar JJ, Haddad MB, Tierney BC, Campbell GL, Marfin AA, Van Gerpen JA, et al. Neurologic manifestations and outcome of West Nile virus infection. JAMA. 2003 Jul 23;290(4):511-5.
- Montgomery RR. Age-related alterations in immune responses to West Nile virus infection. Clin Exp Immunol. 2017 Jan;187(1):26-34.
- Danis K, Papa A, Theocharopoulos G, Dougas G, Athanasiou M, Detsis M, et al. Outbreak of West Nile virus infection in Greece, 2010. Emerg Infect Dis. 2011 Oct;17(10):1868-72.
- Pervanidou D, Detsis M, Danis K, Mellou K, Papanikolaou E, Terzaki I, et al. West Nile virus outbreak in humans, Greece, 2012: third consecutive year of local transmission. Euro Surveill. 2014 Apr 3;19(13).
- Durand B, Tran A, Balanca G, Chevalier V. Geographic variations of the bird-borne structural risk of West Nile virus circulation in Europe. PLoS One. 2017 Oct 12;12(10):e0185962.
- Rudolf I, Betášova L, Blažejová H, Venclíková K, Straková P, Šebesta O, et al. West Nile virus in overwintering mosquitoes, central Europe. Parasit Vectors. 2017 Oct 2;10(1):452.
- Brugman VA, Hernández-Triana LM, England ME, Medlock JM, Mertens PPC, Logan JG, et al. Blood-feeding patterns of native mosquitoes and insights into their potential role as pathogen vectors in the Thames estuary region of the United Kingdom. Parasit Vectors. 2017 Mar 27;10(1):163.
- Fritz ML, Walker ED, Miller JR, Severson DW, Dworkin I. Divergent host preferences of above- and below-ground Culex pipiens mosquitoes and their hybrid offspring. Med Vet Entomol. 2015 Jun;29(2):115-23.
- Osório HC, Zé-Zé L, Amaro F, Nunes A, Alves MJ. Sympatric occurrence of Culex pipiens (Diptera, Culicidae) biotypes pipiens, molestus and their hybrids in Portugal, Western Europe: feeding patterns and habitat determinants. Med Vet Entomol. 2014 Mar;28(1):103-9.
- Vogels CBF, Hartemink N, Koenraadt CJM. Modelling West Nile virus transmission risk in Europe: effect of temperature and mosquito biotypes on the basic reproduction number. Sci Rep. 2017 Jul 10;7(1):5022.
- Pupella S, Pisani G, Cristiano K, Catalano L, Grazzini G. West Nile virus in the transfusion setting with a special focus on Italian preventive measures adopted in 2008-2012 and their impact on blood safety. Blood Transfus. 2013 Oct;11(4):563-74.
- Costa AN, Capobianchi MR, Ippolito G, Palu G, Barzon L, Piccolo G, et al. West Nile virus: the Italian national transplant network reaction to an alert in the north-eastern region, Italy 2011. Euro Surveill. 2011 Oct 13;16(41).
- Centers for Disease Control and Prevention (CDC). Intrauterine West Nile virus infection--New York, 2002. MMWR Morb Mortal Wkly Rep. 2002 Dec 20;51(50):1135-6.
- Centers for Disease Control and Prevention. Possible West Nile virus transmission to an infant through breast-feeding--Michigan, 2002. MMWR Morb Mortal Wkly Rep. 2002 Oct 4;51(39):877-8.
- Hannoun C, Panthier R, Mouchet J, Eouzan JP. Isolation in France of the West Nile Virus from Patients and from the Vector Culex Modestus Ficalbi. C R Hebd Seances Acad Sci. 1964 Nov 30;259:4170-2.
- Venter M, Steyl J, Human S, Weyer J, Zaayman D, Blumberg L, et al. Transmission of West Nile virus during horse autopsy. Emerg Infect Dis. 2010 Mar;16(3):573-5.
- Centers for Disease Control and Prevention (CDC). Laboratory-acquired West Nile virus infections--United States, 2002. MMWR Morb Mortal Weekly Rep. 2002 Dec 20;51(50):1133-5.
- Pérez-Ramírez E, Llorente F, Jiménez-Clavero MÁ. Experimental infections of wild birds with West Nile virus. Viruses. 2014 Feb 13;6(2):752-81.
- Banet-Noach C, Simanov L, Malkinson M. Direct (non-vector) transmission of West Nile virus in geese. Avian Pathol. 2003 Oct;32(5):489-94.
- Bardos V, Adamcova J, Dedei S, Gjini N, Rosicky B, Simkova A. Neutralizing antibodies against some neurotropic viruses determined in human sera in Albania. J Hyg Epidemiol Microbiol Immunol. 1959;3:277-82.
- Joubert L, Oudar J, Hannoun C, Beytout D, Corniou B, Guillon JC, et al. Epidemiology of the West Nile virus: study of a focus in Camargue. IV. Meningo-encephalomyelitis of the horse. Ann Inst Pasteur (Paris). 1970 Feb;118(2):239-47.
- Tsai TF, Popovici F, Cernescu C, Campbell GL, Nedelcu NI. West Nile encephalitis epidemic in southeastern Romania. Lancet. 1998 Sep 5;352(9130):767-71.
- Savage HM, Ceianu C, Nicolescu G, Karabatsos N, Lanciotti R, Vladimirescu A, et al. Entomologic and avian investigations of an epidemic of West Nile fever in Romania in 1996, with serologic and molecular characterization of a virus isolate from mosquitoes. Am J Trop Med Hyg. 1999 Oct;61(4):600-11.
- Tran A, Sudre B, Paz S, Rossi M, Desbrosse A, Chevalier V, et al. Environmental predictors of West Nile fever risk in Europe. Int J Health Geogr. 2014 Jul 1;13:26.
- Papa A, Bakonyi T, Xanthopoulou K, Vazquez A, Tenorio A, Nowotny N. Genetic characterization of West Nile virus lineage 2, Greece, 2010. Emerg Infect Dis. 2011 May;17(5):920-2.
- Platonov AE, Karan LS, Shopenskaia TA, Fedorova MV, Koliasnikova NM, Rusakova NM, et al. Genotyping of West Nile fever virus strains circulating in southern Russia as an epidemiological investigation method: principles and results. Zh Mikrobiol Epidemiol Immunobiol. 2011 Mar-Apr(2):29-37.
- Danis K, Papa A, Papanikolaou E, Dougas G, Terzaki I, Baka A, et al. Ongoing outbreak of West Nile virus infection in humans, Greece, July to August 2011. Euro Surveill. 2011 Aug 25;16(34).
- Lustig Y, Mannasse B, Koren R, Katz-Likvornik S, Hindiyeh M, Mandelboim M, et al. Superiority of West Nile Virus RNA Detection in Whole Blood for Diagnosis of Acute Infection. J Clin Microbiol. 2016 Sep;54(9):2294-7.
- European Commission. West Nile Virus and Blood Safety – Introduction to a preparedness plan in Europe 2012. Based on the EU Satellite Meeting of the Working Group on Blood Safety and WNV, Thessaloniki, 25-26 January 2011 – And on the teleconference, 18 January 2012. Final Working Document 2012 v.2.1. Prepared by: Greece, Italy, Romania and France – 6 June 2012. Available from: https://ec.europa.eu/health/sites/health/files/blood_tissues_organs/docs/wnv_preparedness_plan_2012.pdf.
- Bellini R, Zeller H, Van Bortel W. A review of the vector management methods to prevent and control outbreaks of West Nile virus infection and the challenge for Europe. Parasit Vectors. 2014 Jul 11;7:323.




