The Use of Bovine Colostrum in Sport and Exercise
Abstract
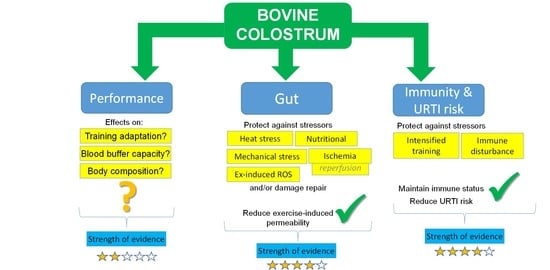
:1. Introduction
2. Studies Related to Physical Performance
2.1. Body Composition and Strength
2.2. High-Intensity and Intermittent Exercise Performance
2.3. Bovine Colostrum Supplementation and Endurance Performance
3. Gastrointestinal Integrity
Bovine Colostrum in Combination with Other Nutrients
4. Immunity and Infection in Athletes
5. Risks and Controversies?
6. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Playford, R.; Weiser, M. Bovine Colostrum: Its Constituents and Uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef]
- McGrath, B.A.; Fox, P.F.; McSweeney, P.L.H.; Kelly, A.L. Composition and properties of bovine colostrum: A review. Dairy Sci. Technol. 2016, 96, 133–158. [Google Scholar] [CrossRef]
- Antonio, J.; Sanders, M.S.; Van Gammeren, D. The effects of bovine colostrum supplementation on body composition and exercise performance in active men and women. Nutrients 2001, 17, 243–247. [Google Scholar] [CrossRef]
- Kerksick, C.; Kreider, R.; Rasmussen, C.; Lancaster, L.; Starks, M.; Greenwood, M.; Milnor, P.; Almada, A.; Earnest, C. Effects of bovine colostrum supplementation on training adaptations II: Perfor-mance. FASEB J. 2001, 15, LB315. [Google Scholar]
- Brinkworth, G.D.; Buckley, J.D.; Slavotinek, J.P.; Kurmis, A.P. Effect of bovine colostrum supplementation on the composition of resistance trained and untrained limbs in healthy young men. Graefe’s Arch. Clin. Exp. Ophthalmol. 2004, 91, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.; Brinkworth, G.; Abbott, M. Effect of bovine colostrum on anaerobic exercise performance and plasma insulin-like growth factor I. J. Sports Sci. 2003, 21, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B. Role of calcium and dairy products in energy partitioning and weight management. Am. J. Clin. Nutr. 2004, 79, 907S–912S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stellingwerff, T.; Bovim, I.M.; Whitfield, J. Contemporary Nutrition Interventions to Optimize Performance in Middle-Distance Runners. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 106–116. [Google Scholar] [CrossRef]
- Stellingwerff, T.; Maughan, R.J.; Burke, L.M. Nutrition for power sports: Middle-distance running, track cycling, rowing, canoeing/kayaking, and swimming. J. Sports Sci. 2011, 29, S79–S89. [Google Scholar] [CrossRef]
- Bishop, D. Dietary Supplements and Team-Sport Performance. Sports Med. 2010, 40, 995–1017. [Google Scholar] [CrossRef]
- Brinkworth, G.D.; Buckley, J.D.; Bourdon, P.C.; Gulbin, J.P.; David, A.Z. Oral bovine colostrum supplementation enhances buffer capacity but not rowing performance in elite female rowers. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Kotsis, Y.; Mikellidi, A.; Aresti, C.; Persia, E.; Sotiropoulos, A.; Panagiotakos, D.B.; Antonopoulou, S.; Nomikos, T. A low-dose, 6-week bovine colostrum supplementation maintains performance and attenuates inflammatory indices following a Loughborough Intermittent Shuttle Test in soccer players. Eur. J. Nutr. 2017, 57, 1181–1195. [Google Scholar] [CrossRef] [Green Version]
- Buckley, J.; Abbott, M.; Brinkworth, G.; Whyte, P. Bovine colostrum supplementation during endurance running training improves recovery, but not performance. J. Sci. Med. Sport 2002, 5, 65–79. [Google Scholar] [CrossRef]
- Shing, C.M. The influence of bovine colostrum supplementation on exercise performance in highly trained cyclists. Br. J. Sports Med. 2006, 40, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Coombes, J.S.; Conacher, M.; Austen, S.K.; Marshall, P.A. Dose effects of oral bovine colostrum on physical work capacity in cyclists. Med. Sci. Sports Exerc. 2002, 34, 1184–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeukendrup, A.E.; Vet-Joop, K.; Sturk, A.; Stegen, J.H.; Senden, J.; Saris, W.H.; Wagenmakers, A.J. Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clin. Sci. 2000, 98, 47–55. [Google Scholar] [CrossRef]
- De Oliveira, E.P.; Burini, R.C.; Jeukendrup, A. Gastrointestinal Complaints During Exercise: Prevalence, Etiology, and Nutritional Recommendations. Sports Med. 2014, 44, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Leon, L.R.; Helwig, B.G. Heat stroke: Role of the systemic inflammatory response. J. Appl. Physiol. 2010, 109, 1980–1988. [Google Scholar] [CrossRef]
- Walter, E.; Gibson, O.R.; Stacey, M.; Hill, N.; Parsons, I.T.; Woods, D. Changes in gastrointestinal cell integrity after marathon running and exercise-associated collapse. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 121, 1179–1187. [Google Scholar] [CrossRef]
- Marchbank, T.; Davison, G.; Oakes, J.R.; Ghatei, M.A.; Patterson, M.; Moyer, M.P.; Playford, R.J. The nutriceutical bovine colostrum truncates the increase in gut permeability caused by heavy exercise in athletes. Am. J. Physiol. Liver Physiol. 2011, 300, G477–G484. [Google Scholar] [CrossRef] [Green Version]
- Davison, G.; Marchbank, T.; March, D.S.; Thatcher, R.; Playford, R.J. Zinc carnosine works with bovine colostrum in truncating heavy exercise–induced increase in gut permeability in healthy volunteers. Am. J. Clin. Nutr. 2016, 104, 526–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- March, D.S.; Marchbank, T.; Playford, R.J.; Jones, A.; Thatcher, R.; Davison, G. Intestinal fatty acid-binding protein and gut permeability responses to exercise. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 117, 931–941. [Google Scholar] [CrossRef] [Green Version]
- March, D.S.; Jones, A.; Thatcher, R.; Davison, G. The effect of bovine colostrum supplementation on intestinal injury and circulating intestinal bacterial DNA following exercise in the heat. Eur. J. Nutr. 2018, 58, 1441–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hałasa, M.; Maciejewska, D.; Baśkiewicz-Hałasa, M.; Machaliński, B.; Safranow, K.; Stachowska, E. Oral Supplementation with Bovine Colostrum Decreases Intestinal Permeability and Stool Concentrations of Zonulin in Athletes. Nutrients 2017, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- McKenna, Z.; Berkemeier, Q.; Naylor, A.; Kleint, A.; Gorini, F.; Ng, J.; Kim, J.-K.; Sullivan, S.; Gillum, T. Bovine colostrum supplementation does not affect plasma I-FABP concentrations following exercise in a hot and humid environment. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 117, 2561–2567. [Google Scholar] [CrossRef]
- Morrison, S.A.; Cheung, S.S.; Cotter, J.D. Bovine colostrum, training status, and gastrointestinal permeability during exercise in the heat: A placebo-controlled double-blind study. Appl. Physiol. Nutr. Metab. 2014, 39, 1070–1082. [Google Scholar] [CrossRef] [PubMed]
- Playford, R.J.; Cattell, M.; Marchbank, T. Marked variability in bioactivity between commercially available bovine colostrum for human use; implications for clinical trials. PLoS ONE 2020, 15, e0234719. [Google Scholar] [CrossRef]
- Hałasa, M.; Maciejewska-Markiewicz, D.; Baśkiewicz-Hałasa, M.; Safranow, K.; Stachowska, E. Post-Delivery Milking Delay Influence on the Effect of Oral Supplementation with Bovine Colostrum as Measured with Intestinal Permeability Test. Medicina 2020, 56, 495. [Google Scholar] [CrossRef]
- Playford, R.J.; Marchbank, T. Oral zinc carnosine reduces multi-organ damage caused by gut ischemia/reperfusion in mice. J. Funct. Foods 2021, 78, 104361. [Google Scholar] [CrossRef]
- Hashem, M.; Hall, C.B. Respiratory syncytial virus in healthy adults: The cost of a cold. J. Clin. Virol. 2003, 27, 14–21. [Google Scholar] [CrossRef]
- Cannon, J.G. Exercise and resistance to infection. J. Appl. Physiol. 1993, 74, 973–981. [Google Scholar] [CrossRef]
- Gleeson, M.; Walsh, N.P. The BASES Expert Statement on Exercise, Immunity, and Infection. J. Sports Sci. 2012, 30, 321–324. [Google Scholar] [CrossRef] [Green Version]
- Schwellnus, M.; Sewry, N.; Snyders, C.; Kaulback, K.; Wood, P.S.; Seocharan, I.; Derman, W.; Hull, J.H.; Valtonen, M.; Jordaan, E. Symptom cluster is associated with prolonged return-to-play in symptomatic athletes with acute respiratory illness (including COVID-19): A cross-sectional study—AWARE study I. Br. J. Sports Med. 2021. [Google Scholar] [CrossRef]
- Engebretsen, L.; Steffen, K.; Alonso, J.M.; Aubry, M.; Dvorak, J.; Junge, A.; Meeuwisse, W.; Mountjoy, M.; Renström, P.; Wilkinson, M. Sports injuries and illnesses during the Winter Olympic Games 2010. Br. J. Sports Med. 2010, 44, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engebretsen, L.; Soligard, T.; Steffen, K.; Alonso, J.M.; Aubry, M.; Budgett, R.; Dvorak, J.; Jegathesan, M.; Meeuwisse, W.H.; Mountjoy, M.; et al. Sports injuries and illnesses during the London Summer Olympic Games 2012. Br. J. Sports Med. 2013, 47, 407–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, D. Medicine at the 2000 Sydney Olympic Games: The New Zealand health team. Br. J. Sports Med. 2002, 36, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valtonen, M.; Waris, M.; Vuorinen, T.; Eerola, E.; Hakanen, A.J.; Mjosund, K.; Grönroos, W.; Heinonen, O.J.; Ruuskanen, O. Common cold in Team Finland during 2018 Winter Olympic Games (PyeongChang): Epidemiology, diagnosis including molecular point-of-care testing (POCT) and treatment. Br. J. Sports Med. 2019, 53, 1093–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Krüger, K.; Nieman, D.C.; Pyne, D.B.; Turner, J.E.; Walsh, N.P. Can exercise af-fect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar] [PubMed]
- Svendsen, I.S.; Taylor, I.M.; Tønnessen, E.; Bahr, R.; Gleeson, M. Training-related and competition-related risk factors for respiratory tract and gastrointestinal infections in elite cross-country skiers. Br. J. Sports Med. 2016, 50, 809–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Albers, R.; Antoine, J.-M.; Bourdet-Sicard, R.; Calder, P.C.; Gleeson, M.; LeSourd, B.; Samartín, S.; Sanderson, I.R.; Van Loo, J.; Dias, F.W.V.; et al. Markers to measure immunomodulation in human nutrition intervention studies. Br. J. Nutr. 2005, 94, 452–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albers, R.; Bourdet-Sicard, R.; Braun, D.; Calder, P.; Herz, U.; Lambert, C.; Lenoir-Wijnkoop, I.; Méheust, A.; Ouwehand, A.; Phothirath, P.; et al. Monitoring immune modulation by nutrition in the general population: Identifying and substantiating effects on human health. Br. J. Nutr. 2013, 110, S1–S30. [Google Scholar] [CrossRef] [Green Version]
- Cox, A.J.; Gleeson, M.; Pyne, D.; Callister, R.; Hopkins, W.G.; Fricker, P.A. Clinical and Laboratory Evaluation of Upper Respiratory Symptoms in Elite Athletes. Clin. J. Sport Med. 2008, 18, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; A Woods, J.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goetz, L.; et al. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar] [PubMed]
- Spence, L.; Brown, W.J.; Pyne, D.; Nissen, M.D.; Sloots, T.P.; Mccormack, J.G.; Locke, A.S.; Fricker, P.A. Incidence, Etiology, and Symptomatology of Upper Respiratory Illness in Elite Athletes. Med. Sci. Sports Exerc. 2007, 39, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Bermon, S.; Castell, L.M.; Calder, P.; Bishop, N.C.; Blomstrand, E.; Mooren, F.C.; Krüger, K.; Kavazis, A.N.; Quindry, J.C.; Senchina, D.S.; et al. Consensus Statement Immunonutrition and Exercise. Exerc. Immunol. Rev. 2017, 23, 8–50. [Google Scholar]
- Hanstock, H.G.; Walsh, N.P.; Edwards, J.P.; Fortes, M.B.; Cosby, S.L.; Nugent, A.; Curran, T.; Coyle, P.V.; Ward, M.D.; Yong, X.H.A. Tear Fluid SIgA as a Noninvasive Biomarker of Mucosal Immunity and Common Cold Risk. Med. Sci. Sports Exerc. 2016, 48, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Kustin, T.; Ling, G.; Sharabi, S.; Ram, D.; Friedman, N.; Zuckerman, N.; Bucris, E.D.; Glatman-Freedman, A.; Stern, A.; Mandelboim, M. A method to identify respiratory virus infections in clinical samples using next-generation sequencing. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Benson, K.F.; Carter, S.G.; Patterson, K.M.; Patel, D.; Jensen, G.S. A novel extract from bovine colostrum whey supports anti-bacterial and anti-viral innate immune functions in vitro and in vivo. Prev. Med. 2012, 54, S116–S123. [Google Scholar] [CrossRef]
- Sugisawa, H.; Itou, T.; Saito, M.; Moritomo, T.; Miura, Y.; Sakai, T. A low-molecular-weight fraction of bovine colostrum and milk enhances the oxidative burst activity of polymorphonuclear leukocytes. Vet. Res. Commun. 2003, 27, 453–461. [Google Scholar] [CrossRef]
- Rusu, D.; Drouin, R.; Pouliot, Y.; Gauthier, S.; Poubelle, P.E. A Bovine Whey Protein Extract Stimulates Human Neutrophils to Generate Bioactive IL-1Ra through a NF-κB- and MAPK-Dependent Mechanism. J. Nutr. 2009, 140, 382–391. [Google Scholar] [CrossRef] [Green Version]
- Shing, C.M.; Peake, J.M.; Suzuki, K.; Jenkins, D.G.; Coombes, J.S. Bovine Colostrum Modulates Cytokine Production in Human Peripheral Blood Mononuclear Cells Stimulated with Lipopolysaccharide and Phytohemagglutinin. J. Interf. Cytokine Res. 2009, 29, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Vecchi, A.; Mantegani, P.; Mantelli, B.; Fortis, C.; Lazzarin, A. Immunomodulatory effects of bovine colostrum in human peripheral blood mononuclear cells. New Microbiol. 2007, 30, 447–454. [Google Scholar] [PubMed]
- Sugisawa, H.; Itou, T.; Ichimura, Y.; Sakai, T. Bovine Milk Enhances the Oxidative Burst Activity of Polymorphonuclear Leukocytes in Low Concentrations. J. Vet. Med Sci. 2002, 64, 1113–1116. [Google Scholar] [CrossRef] [Green Version]
- Davison, G. Bovine Colostrum and Immune Function after Exercise. Pediatric Fitness 2012, 59, 62–69. [Google Scholar] [CrossRef]
- Jensen, G.S.; Patel, D.; Benson, K.F. A novel extract from bovine colostrum whey supports innate immune functions. II. Rapid changes in cellular immune function in humans. Prev. Med. 2012, 54, S124–S129. [Google Scholar] [CrossRef]
- Crooks, C.; Wall, C.; Cross, M.L.; Rutherfurd-Markwick, K.J. The effect of bovine colostrum supplementation on salivary IgA in distance runners. J. Sport 2006, 16, 47–64. [Google Scholar] [CrossRef]
- Mero, A.; Kähkönen, J.; Nykänen, T.; Parviainen, T.; Jokinen, I.; Takala, T.; Nikula, T.; Rasi, S.; Leppäluoto, J. IGF-I, IgA, and IgG responses to bovine colostrum supplementation during training. J. Appl. Physiol. 2002, 93, 732–739. [Google Scholar] [CrossRef] [Green Version]
- Davison, G.; Diment, B.C. Bovine colostrum supplementation attenuates the decrease of salivary lysozyme and enhances the recovery of neutrophil function after prolonged exercise. Br. J. Nutr. 2009, 103, 1425–1432. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.; Cameron, S.J.; Thatcher, R.; Beecroft, M.S.; Mur, L.A.; Davison, G. Effects of bovine colostrum supplementation on upper respiratory illness in active males. Brain Behav. Immun. 2014, 39, 194–203. [Google Scholar] [CrossRef]
- Crooks, C.; Cross, M.L.; Wall, C.; Ali, A. Effect of Bovine Colostrum Supplementation on Respiratory Tract Mucosal Defenses in Swimmers. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Patıroğlu, T.; Kondolot, M. The effect of bovine colostrum on viral upper respiratory tract infections in children with immunoglobulin A deficiency. Clin. Respir. J. 2011, 7, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Shing, C.M.; Peake, J.M.; Suzuki, K.; Jenkins, D.G.; Coombes, J.S. A pilot study: Bovine colostrum supplementation and hormonal and autonomic responses to competitive cycling. J. Sports Med. Phys. Fit. 2013, 53, 490–501. [Google Scholar]
- Shing, C.M.; Peake, J.; Suzuki, K.; Okutsu, M.; Pereira, R.; Stevenson, L.; Jenkins, D.G.; Coombes, J.S. Effects of bovine colostrum supplementation on immune variables in highly trained cyclists. J. Appl. Physiol. 2007, 102, 1113–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Główka, N.; Durkalec-Michalski, K.; Woźniewicz, M. Immunological Outcomes of Bovine Colostrum Supplementation in Trained and Physically Active People: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 1023. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Tuomola, E.; Arvilommi, H.; Salminen, S. Modulation of human humoral immune response through orally adminis-tered bovine colostrum. FEMS Immunol. Med. Microbiol. 2001, 31, 93–96. [Google Scholar] [CrossRef]
- Wolvers, D.A.W.; Van Herpen-Broekmans, W.M.R.; Logman, M.H.G.M.; Van Der Wielen, R.P.J.; Albers, R. Effect of a mixture of micronutrients, but not of bovine colostrum concentrate, on immune function parameters in healthy volunteers: A randomized placebo-controlled study. Nutr. J. 2006, 5, 28. [Google Scholar] [CrossRef] [Green Version]
- Satyaraj, E.; Reynolds, A.; Pelker, R.; Labuda, J.; Zhang, P.; Sun, P. Supplementation of diets with bovine colostrum influences immune function in dogs. Br. J. Nutr. 2013, 110, 2216–2221. [Google Scholar] [CrossRef] [Green Version]
- Cesarone, M.R.; Belcaro, G.; Di Renzo, A.; Dugall, M.; Cacchio, M.; Ruffini, I.; Pellegrini, L.; Del Boccio, G.; Fano, F.; Ledda, A.; et al. Prevention of Influenza Episodes With Colostrum Compared With Vaccination in Healthy and High-Risk Cardiovascular Subjects. Clin. Appl. Thromb. 2007, 13, 130–136. [Google Scholar] [CrossRef]
- Brinkworth, G.D.; Buckley, J.D. Concentrated bovine colostrum protein supplementation reduces the incidence of self-reported symptoms of upper respiratory tract infection in adult males. Eur. J. Nutr. 2003, 42, 228–232. [Google Scholar] [CrossRef]
- Jones, A.W.; March, D.S.; Curtis, F.; Bridle, C. Bovine colostrum supplementation and upper respiratory symptoms during exercise training: A systematic review and meta-analysis of randomised controlled trials. BMC Sports Sci. Med. Rehabil. 2016, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, A.; March, D.S.; Thatcher, R.; Diment, B.; Walsh, N.P.; Davison, G. The effects of bovine colostrum supplementation on in vivo immunity following prolonged exercise: A randomised controlled trial. Eur. J. Nutr. 2019, 58, 335–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.D.H.; Coakley, S.L.; Ward, M.D.; Macfarlane, A.W.; Friedmann, P.S.; Walsh, N.P. Exercise-induced stress inhibits both the induction and elicitation phases of in vivo T-cell-mediated immune responses in humans. Brain Behav. Immun. 2011, 25, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Diment, B.C.; Fortes, M.B.; Edwards, J.P.; Hanstock, H.G.; Ward, M.D.; Dunstall, H.M.; Friedmann, P.S.; Walsh, N.P. Exercise Intensity and Duration Effects on In Vivo Immunity. Med. Sci. Sports Exerc. 2015, 47, 1390–1398. [Google Scholar] [CrossRef] [Green Version]
- Davison, G.; Kehaya, C.D.B.; Diment, B.C.; Walsh, N.P. Carbohydrate supplementation does not blunt the prolonged exercise-induced reduction of in vivo immunity. Eur. J. Nutr. 2015, 55, 1583–1593. [Google Scholar] [CrossRef] [Green Version]
- Davison, G.; Jones, A.W.; Marchbank, T.; Playford, R.J. Oral bovine colostrum supplementation does not increase circulating insulin-like growth factor-1 concentration in healthy adults: Results from short- and long-term administration studies. Eur. J. Nutr. 2019, 59, 1473–1479. [Google Scholar] [CrossRef] [Green Version]
- Duff, W.R.; Chilibeck, P.D.; Rooke, J.J.; Kaviani, M.; Krentz, J.R.; Haines, D.M. The Effect of Bovine Colostrum Supplementation in Older Adults During Resistance Training. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 276–285. [Google Scholar] [CrossRef]
- Mero, A.; Miikkulainen, H.; Riski, J.; Pakkanen, R.; Aalto, J.; Takala, T. Effects of bovine colostrum supplementation on serum IGF-I, IgG, hormone, and saliva IgA during training. J. Appl. Physiol. 1997, 83, 1144–1151. [Google Scholar] [CrossRef]
- Willoughby, D.S.; Stout, J.R.; Wilborn, C.D. Effects of resistance training and protein plus amino acid supplementation on muscle anabolism, mass, and strength. Amino Acids 2006, 32, 467–477. [Google Scholar] [CrossRef]
- Giovannucci, E.; Pollak, M.; Liu, Y.; A Platz, E.; Majeed, N.; Rimm, E.B.; Willett, W.C. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiology Biomarkers Prev. 2003, 12, 84–89. [Google Scholar]
- Kuipers, H.; Van Breda, E.; Verlaan, G.; Smeets, R. Effects of oral bovine colostrum supplementation on serum insulin-like growth factor-i levels. Nutrients 2002, 18, 566–567. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davison, G. The Use of Bovine Colostrum in Sport and Exercise. Nutrients 2021, 13, 1789. https://doi.org/10.3390/nu13061789
Davison G. The Use of Bovine Colostrum in Sport and Exercise. Nutrients. 2021; 13(6):1789. https://doi.org/10.3390/nu13061789
Chicago/Turabian StyleDavison, Glen. 2021. "The Use of Bovine Colostrum in Sport and Exercise" Nutrients 13, no. 6: 1789. https://doi.org/10.3390/nu13061789