Abstract
Sleep inertia is a disabling state of grogginess and impaired vigilance immediately upon awakening. The adenosine receptor antagonist, caffeine, is widely used to reduce sleep inertia symptoms, yet the initial, most severe impairments are hardly alleviated by post-awakening caffeine intake. To ameliorate this disabling state more potently, we developed an innovative, delayed, pulsatile-release caffeine formulation targeting an efficacious dose briefly before planned awakening. We comprehensively tested this formulation in two separate studies. First, we established the in vivo caffeine release profile in 10 young men. Subsequently, we investigated in placebo-controlled, double-blind, cross-over fashion the formulation’s ability to improve sleep inertia in 22 sleep-restricted volunteers. Following oral administration of 160 mg caffeine at 22:30, we kept volunteers awake until 03:00, to increase sleep inertia symptoms upon scheduled awakening at 07:00. Immediately upon awakening, we quantified subjective state, psychomotor vigilance, cognitive performance, and followed the evolution of the cortisol awakening response. We also recorded standard polysomnography during nocturnal sleep and a 1-h nap opportunity at 08:00. Compared to placebo, the engineered caffeine formula accelerated the reaction time on the psychomotor vigilance task, increased positive and reduced negative affect scores, improved sleep inertia ratings, prolonged the cortisol awakening response, and delayed nap sleep latency one hour after scheduled awakening. Based on these findings, we conclude that this novel, pulsatile-release caffeine formulation facilitates the sleep-to-wake transition in sleep-restricted healthy adults. We propose that individuals suffering from disabling sleep inertia may benefit from this innovative approach.
Trials registration: NCT04975360.
Similar content being viewed by others
Introduction
Sleep inertia is a disabling state of reduced physical and mental drive following awakening1, which typically lasts for less than 30 min but symptoms may persist for several hours in susceptible individuals2,3,4. A large portion of healthy adolescents report persistent difficulties to rise in the morning5 and many shift and on-call night workers exhibit impaired performance and grogginess after awakening, which raises important safety concerns in operational settings6,7. Furthermore, impaired post-awakening vigilance and mood is highly prevalent in a broad range of neurological and psychiatric conditions7,8,9,10.
Several biological and environmental factors influence the manifestation of sleep inertia. For example, abrupt awakening from deep sleep (also referred to as slow-wave sleep or stage N3 of non-rapid-eye-movement [NREM] sleep) is associated with more severe sleep inertia symptoms when compared to awakening from more superficial NREM sleep and rapid-eye-movement (REM) sleep11,12. This finding may suggest that neurophysiological processes underlying sleep and particularly deep NREM sleep carry over into wakefulness and contribute to behavioral and cognitive deficits associated with sleep inertia13. The proportion of deep sleep and the odds of awakening from N3 sleep is increased when a sleep opportunity is shorted such as in sleep restriction. Accordingly, abrupt awakening during sleep restriction enhances sleep inertia symptoms4.
The neuromodulator adenosine is a key regulator of deep sleep14 and adenosinergic neuromodulation may play an essential role in the manifestation of sleep inertia15. Consistent with this view, the adenosine receptor antagonist, caffeine, is widely used to counteract sleep inertia. Besides attenuating sleepiness and deficits in vigilance16,17, caffeine augments cardiovascular and respiratory functions18 and promotes the release of cortisol, a key hormone of the hypothalamic–pituitary–adrenal (HPA) axis19. The HPA axis regulates several psycho-vegetative aspects of the wake-up process, including the cortisol awakening response (CAR). The CAR reflects HPA axis function20,21 and may be associated with the propensity of sleep inertia22.
Because the impairments are most severe immediately upon awakening, proactive strategies aiming for optimal sleep length and timing have been recommended to minimize sleep inertia symptoms6. Nevertheless, it is not always possible to plan and obtain sleep of sufficient length and quality and at the optimal time of day. On the other hand, currently there exists no convincing evidence that reactive countermeasures to sleep intertia, i.e. strategies implemented upon wake-up, are sufficiently effective6. Although caffeine is the best available option, coffee takes 20–30 min to have an alerting effect23. The bioavailability of reactive oral caffeine intake prevents effective amelioration of sleep inertia symptoms for at least 12–18 min upon waking17, unless it is taken as a proactive countermeasure prior to a short sleep or nap bout16,24. When taken before sleep, however, caffeine can delay sleep onset, reduce total sleep time and attenuate the amount of deep slow wave sleep when a pharmacologically active concentration is present in the organism during sleep24,25.
The time-controlled administration of pharmaceuticals in accordance with the sleep–wake cycle provides an essential pillar in the emerging concept of chronopharmacology and chronotherapeutics26,27. We aimed at developing a chronotherapeutic caffeine formulation that ameliorates impaired subjective state, vigilance and performance immediately upon awakening without disturbing the quality of the preceeding sleep episode. For this purpose, we invented a delayed, pulsatile-release caffeine delivery system targeted to reach an efficacious plasma concentration approximately 7 h after intake. When ingested at habitual bedtime, we hypothesized that this formula would improve vigilance and mood, elevate the CAR, and reduce sleep propensity on the subsequent morning after wake-up from nocturnal sleep. We tested these hypotheses in two separate studies. First, we examined the in vivo release properties of the engineered caffeine formula throughout a nocturnal sleep episode. Then, we comprehensively investigated in randomized, double-blind, cross-over, placebo-controlled manner its effects on behavioral, emotional, neurocognitive and physiological symptoms of sleep inertia in sleep-restricted healthy young men.
Methods
Participants and permission
A total of 32 healthy young men (mean age: 25.6 ± 3.7 years) participated in the two studies (in vivo validation study: n = 10; pharmacodynamic study: n = 22), whereof 5 subjects participated in both experiments. The following criteria were required for inclusion: (i) male sex in order to avoid the potential impact of menstrual cycle on sleep physiology or HPA axis activity, (ii) age within the range of 18–34 years, (iii) a body-mass-index below 25, (iv) an Epworth Sleepiness Score (ESS) below 10, (v) habitual sleep onset latency below 20 min, (vi) regular sleep–wake rhythm with bedtime between 10 pm and 1 am, (vii) absence of any somatic or psychiatric disorders, (viii) no acute or chronic medication intake, (ix) non-smoking, (x) no history of drug abuse (lifetime use > 5 occasions, with exception of occasional cannabis use), and (xi) caffeine consumption of less than 4 units per day (coffee, tea, chocolate, cola, energy drinks).
The participants were instructed to abstain from illicit drugs and caffeine during the entire study, starting two weeks prior to the first experimental night until the end of the study (the day after the second experimental night). No alcohol was allowed 24 h before the expermental nights. The minimal wash out period before the experimental nights was 7 days. Participants were also instructed to keep an individual regular sleep–wake rhythm (23:00–07:00 or 22:00–06:00 depending on the volunteers’ habitual bedtime) during the entire study, starting 2 weeks prior to the experimental night. All included participants chose to keep either a 22:00–06:00 rhythm or a 23:00–07:00 rhythm. To ensure adherence to the regular sleep–wake pattern, participants were instructed to wear a rest-activity monitor on the non-dominant arm and to keep a sleep–wake diary.
The studies were approved by the Cantonal Ethics Committee of the Canton of Zurich (BASEC: 2018-00533) and registered on ClinicalTrials.gov (Identifier: NCT04975360). All participants provided written informed consent according to the declaration of Helsinki.
Study drug
The caffeine pulsatile-release formulation was manufactured using a drug layering process28. The details of the engineering and manufacturing processes will be reported elsewhere. In brief, caffeine and the excipients were dispersed in the coating media and then sprayed onto inert microcrystalline cellulose spheres using a fluid bed through a Wurster tube with continuous inlet air that dries the liquid in the dispersion, to obtain various layers consisting of caffeine and release-controlling polymers. The applied release-controlling polymeric system was based on methacrylate copolymers, which control the release of caffeine in both a pH-dependent and pH-independent manner29,30. Thereby, the release mechanism of the polymeric system was mainly driven by the swellability and permeability of the copolymers31. The final micropellets were then encapsulated into hydroxy-propyl-methylcellulose capsules.
To evaluate the in vitro dissolution profiles of the manufactured formula, different prototypes were tested by means of state-of-the-art dissolution assays, mimicking gastrointestinal conditions32,33. Development and in vitro testing of the caffeine pulsatile-release formulation and placebos was conducted at Elixir Pharmaceutical Research and Development Corporation in Ankara. For the in vivo study, the most suited prototype with a favorable in vitro dissolution profile was chosen.
In vivo validation study
In a first open-label evaluation study of the engineered delivery system, the in vivo caffeine release profile was determined in 10 fasted (no food or beverage consumption 2 h before drug administration) male individuals. After oral intake at 22:30, study participants were allowed to sleep from 23:00 to 07:00, while blood was continuously sampled. Samples were collected from the left antecubital vein at baseline (22:00), and 1.5, 2.5, 3.5, 4.5, 5.5, 6.5, 7.5, 8.5, 9.5, 10.5, 13.5 and 17.5 h after drug administration. During the sleep episode in the soundproof and climatized bedrooms of the sleep laboratory, the venous catheter was connected to a blood-collection setup in an adjacent room (Heidelberger plastic tube extensions through the wall). Thus, blood samples (4 ml, BD Vacutainer EDTA) were collected without disturbing the sleeping study participants. The intravenous line was kept patent with a slow drip (10 ml/h) of heparinized saline (1000 IU heparin in 0.9 g NaCl/dl; HEPARIN Bichsel; Bichsel AG, 3800 Unterseen, Switzerland). Blood samples were immediately centrifuged for 10 min at 2000 RCF and plasma samples were immediately stored on ice until final storage at − 80 °C.
Pharmacodynamic study
In the pharmacodynamic study, the pulsatile-release caffeine formulation (which was validated in vivo as described above) or a placebo (matched in appearance) were administered 8.5 h before the scheduled wake-up time to the fasted participants (no food or beverage consumption 2 h before drug administration). To exacerbate sleep inertia symptoms and avoid neuropsychological ceiling/floor effects, which are frequent in intervention studies with highly functioning healthy volunteers, all participants were sleep restricted. More specifically, they were kept awake until 02:00 (participants adhering to a 22:00–06:00 rhythm) or 03:00 (23:00–07:00 rhythm), then given a 4-h sleep opportunity, and awoken at 06:00 (22:00–06:00 rhythm) or 07:00 (23:00–07:00 rhythm). At 3.5 h post-administration, all volunteers received a standardized, light meal. Blood was continuously sampled upon drug administration and the caffeine release from the formulation was monitored as described above. Upon awakening, the effects of the formulation on neurobehavioral, emotional, cognitive, and endocrinological markers of sleep inertia were assessed. Additionally, physiological sleep tendency was investigated by determining the sleep characteristics of a 1-h nap opportunity starting 1 h post-awakening. To simplify descriptions and data presentations, we will only refer to the 23:00–07:00 rhythm with respect to the time-points of the tasks, because only a small minority of the participants followed the 22:00–06:00 rhythm.
The pharmacodynamic study followed a randomized, double-blind, placebo-controlled, crossover design with a wash-out period of at least 1 week between the caffeine and placebo conditions.
The details of both study designs are illustrated in Fig. 1.
Illustration of the study procedures of the in vivo validation study (A) and the pharmacodynamic study (B). Timepoints of blood withdrawal are indicated as grey drops (drop symbols). Sleep episodes are highlighted as hatched areas. In both studies, sleep was continuously recorded by polysomnography. In the pharmacodynamic study, participants were kept awake until 3:00. Immediately after awakening from a restricted 4-h nocturnal sleep episode (at 07:00), volunteers performed a 1-h testing battery (referred to as “Testing”) to quantify behavioral, cognitive, emotional and physiological markers of sleep inertia. At 8:00, the participants were given a 1-h nap opportunity, while the latency to fall asleep and the sleep profile were recorded with polysomnography. Sleep inertia assessment, CAR measurements and physiological sleepiness testing were only performed in the pharmacodynamic study.
Analysis of caffeine levels
Caffeine and caffeine-13C3 were purchased from Sigma-Aldrich (St. Louis, USA). All chemicals used were of the highest purification grade available. Briefly, 200 µl of plasma, 50 µl of the internal standard (IS) (40 µM caffeine-13C3) and 50 µl of methanol (MeOH) were added to an Eppendorf tube. For protein precipitation, 400 µl of acetonitrile (ACN) were slowly added. Samples were shaken for 10 min and centrifuged at 10,000 rpm for 5 min. A volume of 350 µl of the supernatant was transferred into an auto-sampler vial and evaporated to dryness under a gentle stream of nitrogen. For reconstitution, 250 µl of an eluent-mixture (95:5, v/v) was added. Quality control (QC) samples and calibrators (Cal) were prepared with the same sample preparation, replacing the 50 µl of MeOH with the Cal or QC solutions. The plasma samples were analyzed on an ultra-high performance liquid chromatography (UHPLC) system (Thermo Fisher, San Jose, CA, USA) coupled to a linear ion trap quadrupole mass spectrometer 5500 (Sciex, Darmstadt, Germany). The mobile phases of the UHPLC consisted of water (eluent A) and a mixture (70:30 v/v) of MeOH and ACN (eluent B), both containing 0.1% of formic acid (v/v). A volume of 5 µl of the prepared samples was used for quantification. Using a Kinetex Biphenyl column (50 × 2.1 mm, 1.7 µm) (Phenomenex, Aschaffenburg, Germany), the flow rate was set to 0.45 ml/min with the following gradient: start conditions 95% of eluent A, decreasing to 80% in 3 min followed by a quick decrease to 2% within 0.5 min. These conditions were held for 1 min and switched to the starting conditions for re-equilibration for 1 min. The mass spectrometer was operated in positive electrospray ionization mode with scheduled multiple reaction monitoring. Three MRM transitions were used for both analytes. For quantification, the peak area of the analytes was further integrated and divided by the peak area of the IS. Cal samples were fitted with a least-squares fit and weighted by 1/x. The limit of quantification of caffeine was 1.2 µM.
Testing of neurobehavioral performance and subjective state
A comprehensive test battery including the following tasks, validated questionnaires, and physiological measures to assess behavioral, subjective, emotional, cognitive and endocrinological markers of sleep inertia was administered immediately upon awakening (07:00–08:00).
Psychomotor vigilance test (PVT)
Vigilance was assessed with a 10-min version of the PVT34 at 02:30 and 07:05 am, i.e. immediately after awakening. The median reaction time (RT) and the numbers of lapses (trials with RT > 500 ms) were analyzed.
Positive and negative affective schedule (PANAS)
The PANAS35 was used to assess mood 15 min after awakening (at 07:15 am).
Caffeine acute questionnaire (CAQ)
To assess specific caffeine-related effects, the CAQ36 was applied 30 min after awakening. Participants were asked to rate the following items on a 5-point scale (not at all, a little bit, moderate, much, very much); “Are you feeling any of these caffeine-related effects”: “increased vigilance”, “increased wellbeing”, “increased heart rate”, “increased sociability”, “increased motivation to work”, “increased tension”, “increased concentration”, “increased urge to urinate”, “increased shakiness”, “reduced appetite”, “increased energy”, “increased sweating”, “increased self-confidence”, “reduced headache”, “increased anxiety”, “reduced tiredness”, “stimulation”, “increased nervousness”, “reduced boredom” and “increased stomach trouble”.
Modified sleep inertia questionnaire (SIQ)
We modified the SIQ37 to assess volunteers’ subjective experience of the awakening process. The original version of the SIQ represents a trait inventory, in which participants are instructed to rate the quality of their awakening process during the last week, namely on physiological, emotional, cognitive and behavioral levels. Thereby, the inventory instruction reads as follows: “On a typical morning in the past week, after you woke up, to what extent did you, for example, have problems to get out of bed” (possible ratings: 1 = not at all, 2 = a little, 3 = somewhat, 4 = often, 5 = all the time). For the present study, we rephrased the inventory’s instruction to gain state information of the wake-up process of the experimental morning (rather than trait information of the last week), to analyze the acute effects of our pulsatile-release formula. The instruction was rephrased as follows: “How strong did you feel the following aspects after you woke up this morning compared to a normal morning last week: for example, have problems to get out of bed” (possible ratings: − 3 = extremely less, − 2 = much less, − 1 = a little bit less, 0 = same, 1 = a little bit more, 2 = much more, 3 = extremely more). For our purpose, the modified version of the SIQ was renamed to Acute Sleep Inertia Questionnaire (ASIQ) and was administered at 07:45.
N-back task
At 7:47 the n-back task was executed in 1-, 2- and 3-back versions38. Over a period of 7 min, a random series of letters were displayed and subjects were instructed to press a key when the currently displayed letter corresponded to the previous (1-back), the penultimate (2-back) or the antepenultimate (3-back) letter, respectively. The reaction times and the number of correct and incorrect answers were assessed.
d2-task
Finally, the d2-task, a neuropsychological measure of selective and sustained attention and visual scanning speed39, was administered at 07:55. In this task, participants were instructed to cross out any letter “d” with two marks above it or below it in any order. The surrounding distractors were either a “p” with two marks or a “d” with one or three marks. For each line (14 lines in total), subjects were given 20 s to mark all “d” with two marks and then instructed to proceed to the next line. The number of correct and incorrectly crossed characters were determined.
All questionnaires as well as the n-back and d2 tasks were administered as paper–pencil versions.
Cortisol awakening response (CAR)
Cortisone-D7 was purchased from Sigma Aldrich (Buchs, Switzerland) and 13C3-cortisol was purchased from Isoscience (Ambler, USA). Saliva of each subject was sampled at time points 07:00 (immediately after awakening), 07:15, 07:30, 07:45, and 08:00. Participants were instructed to chew the swab for 60 s and then return it into the Salivette® tube (Sarstedt, Germany). After sampling, tubes were immediately stored on ice until final storage at − 80 °C. For cortisol detection, tubes were defrosted and centrifuged for 5 min at 5000 rpm to yield clear saliva in the conical tube. Two subjects had to be excluded, as the amount of saliva yielded from the swabs was insufficient. Then, the swab was removed and the yielded saliva was spiked with 50 μl IS (0.1 ng/μl Cortison-d7) for further analysis. A fully automated supported liquid extraction (SLE) was carried out by transferring 265 μl saliva into a column rack (24 × 6 ml) from Biotage® Extrahera (Biotage, Uppsala, Sweden) and adding 300 μl water to the sample. After mixing the extracts were automatically loaded onto Isolute SLE + columns and allowed to absorb for 5 min. Analytes were then eluted two times with 1.5 ml ethyl acetate with a waiting time of 5 min in-between. The extracts were dried in a Turbovap® (Biotage, Uppsala, Sweden) at 35 °C. The dry residues were resuspended using 150 μl methanol and 350 μl ammonium formate (5 mM) solution, which was used for liquid chromatography-tandem mass spectrometry (LC–MS/MS) analysis following a recently published method using 13C3-labeled cortisol as surrogate analyte for calibration40. The saliva samples were analyzed on an LC–MS/MS system that consisted of a Shimadzu Prominence UFLC (Shimadzu, Kyoto, Japan) high pressure liquid-chromatography (HPLC) system coupled to a Sciex QTRAP® 6500 + linear ion trap quadrupole mass spectrometer (Sciex, Darmstadt, Germany). 10 μl of the samples were injected onto a Phenomenex® Kinetex® C18 column (2.6 μm, 50 × 2.10 mm). The mobile phase consisted of 10 ml ammonium formate (1 M) and 2 ml formic acid in 2 l water (A) and 10 ml ammonium formate (1 M) in 2 l methanol (B). The flow rate was 0.3 ml/min and the temperature of the column oven was set to 40 °C. The quantification was achieved by using the mass spectrometer in multiple reaction monitoring (MRM) with an ion spray voltage of − 4500 V. Cortisol was measured as formic acid adduct [(M–H) + 46]− in negative electrospray ionization mode. The method was validated according to the guidelines of the German Society of Toxicology and Forensic Chemistry (GTFCh). The calibration was prepared by adding 13C3-cortisol to saliva in the concentration range of 0.55 nmol/ml up to 55 nmol/ml. QC samples were prepared in low concentrations (1.5 nmol/l). The limit of detection for cortisol was 0.55 nmol/l and the limit of quantification was 1.1 nmol/l.
Polysomnography
As in previous studies41,42,43,44, sleep in vivo from 03:00 to 07:00 in the pharmacodynamic study was quantified by all-night polysomnography with Rembrandt® Datalab (Version 8; Embla Systems, Planegg, Germany). in vivo Sleep pressure after awakening was also assessed by determining the participants’ sleep onset latency (SOL) and sleep patterns during a nap opportunity starting at 08:00 (Fig. 1). The recording setup consisted of 10 EEG electrodes (Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, 02) according to the 10–20 system45, a bipolar electrooculogram (EOG), a submental electromyogram (EMG) and an electrocardiogram (ECG). The individual EEG electrode coordinates were marked by cutting a few hairs at the electrode positions, to ensure that the electrodes were placed at the very same place in both experimental conditions.
All data were recorded with dedicated polysomnographic amplifiers (Artisan®, Micromed, Mogliano Veneto, Italy). As in previous studies of the lab (Dornbierer et al. 2019), the analog signals were conditioned by a high-pass filter (EEG: − 3 dB at 0.15 Hz; EMG: 10 Hz; ECG: 1 Hz) and an antialiasing low-pass filter (− 3 dB at 67.2 Hz), digitized and stored with a resolution of 256 Hz (sampling frequency of 256 Hz). The data of one participant were excluded from the nocturnal sleep analyses, due to insufficient EEG quality.
Visual sleep stage scoring
Sleep variables were visually scored based on 30-s epochs according to the criteria of the American Academy of Sleep Medicine46. For sleep scoring, the C3-A2 derivation was used. Movement- and arousal-related artifacts were visually identified and excluded from the analyses. The following sleep variables were computed: time spent in (i) wakefulness (Wake), (ii) stage 1 (N1), (iii) stage 2 (N2), (iv) stage 3 (N3), (v) stage REM sleep, (vi) sleep onset latency (SOL = time between lights-off and first occurrence of N1), (vii) sleep efficiency (SEFF = [TST⁄TIB]*100%; TST = time spent in N1, N2, N3 and REM sleep; TIB = time between lights-off and lights-on), and (viii) pre-awakening stages wake, N1, N2, N3 and REM sleep.
Statistical analyses
Independent linear mixed-effects models, with condition (caffeine vs. placebo) and time point (02:30 and 07:05 for PVT analyses; 07:00, 07:15, 07:30, 07:45 and 08:00 for CAR analyses) as within-subject factors, and subject ID as random effect were employed on ‘R’ for the analysis of the (1) PVT; (2) PANAS; (3) CAQ; (4) ASIQ; (5) n-back; (6) d2 task; (7) CAR; (8) nocturnal sleep variables; and (9) morning nap variables (RStudio Version 1.0.136; RStudio, Inc.; R-package “lme4,” Version 1.1–15). For all applied models, normal Q-Q plots were applied, demonstrating normality of the residuals. Moreover, the assumption of homoscedasticity and linearity was verified using a Tukey-Anscombe plot (residuals vs. fitted). Post-hoc testing was carried out using the ‘R’ package emmeans (Version 1.2.1). The p values of the post-hoc tests were corrected for multiple comparison using Benjamini–Hochberg correction of the false discovery rate47. If not noted otherwise, only significant effects and differences are reported.
Results
Caffeine release profiles
The in vivo validation study during sleep revealed a pulsatile-release profile of the administered formulation; cmax (maximal plasma concentration) was reached after 10.5 h (Fig. 2A). The caffeine curve followed a sustained-release profile, and efficacious plasma levels (> 5 μM) were attained after 7 h48,49.
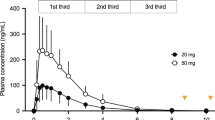
Evolution of the caffeine plasma concentration over time for the in vivo validation study (A) and the pharmacodynamic study (B). Black dots indicate mean caffeine plasma concentrations, error bars indicate standard errors (SEM). The horizontal dashed line at 5 μM indicates the threshold concentration of caffeine efficacy. Time point ‘0’ on the x-axis refers to 22:30 when the caffeine was administered. Sleep periods are indicated as hatched areas.
Unexpectedly, in the pharmacodynamic study, the caffeine release profile distinctly differed from that in the in vivo validation study. A sustained-release of caffeine started after 3.5 h and efficacious plasma caffeine levels were already attained 5 h post-administration (Fig. 2B). The premature burst was most likely triggered by gastric movements due to a standardized meal that was served to the study participants 3.5 h post-administration in the pharmacodynamic study. This meal was absent in the in vivo validation study.
Neurobehavioral, subjective, emotional and physiological symptoms of sleep inertia
Psychomotor vigilance test (PVT)
The statistical analyses of the PVT data revealed a significant condition * timepoint interaction for median reaction time (F = 29.45; p < 0.001), such that caffeine improved the PVT median response time by roughly 10 ms when compared to placebo (Fig. 3). On the other hand, the number of lapses remained unaffected (F = 1.04; p = 0.359).
Positive–negative affect Scale (PANAS)
Statistical analyses of the PANAS ratings revealed a significant condition * item interaction (F = 8.49; p = 0.004; η2 = 0.118), such that the engineered caffeine formulation increased positive ratings (p < 0.01) and tended to reduce negative ratings (p = 0.067) when compared to placebo (Fig. 4).
Post-awakening (7:15–8:00) assessments of subjective state. ASIQ = Acute Sleep Inertia Questionnaire (administered at 07:45). CAQ = Caffeine Acute Questionnaire. PANAS-positive = positive affective scale (administered at 07:15). PANAS-negative = negative affective scale (administered at 07:30). *p < 0.05; **p < 0.01 (Benjamini–Hochberg corrected).
Caffeine acute questionnaire (CAQ)
The statistical analyses revealed significantly increased CAQ ratings in the caffeine condition when compared to placebo (F = 5.14; p = 0.034; η2 = 0.196; Fig. 4).
Acute sleep inertia questionnaire (ASIQ)
The statistical analyses revealed a significant condition effect (F = 31.21; p < 0.001; η2 = 0.175), such that the engineered caffeine-release formula reduced the ratings on all subscales of the ASIQ (behavioral, cognitive, emotional; physiological; Fig. 4). Remarkably, several individuals reported less problems to rise from bed on the experimental mornings compared to a normal morning in the week preceding the experiment (indicated as negative values in Fig. 4). This notion was particularly true during the caffeine condition (n = number of subjects with negative values: behavioral n = 9, cognitive n = 12, emotional n = 9, and physiological n = 10) and to a minor degree also during the placebo condition (behavioral n = 5, cognitive n = 7, emotional n = 5, and physiological n = 2).
N-back and d2 tasks
The statistical analyses of the n-back working memory (F = 0.43; p > 0.05) and the d2 sustained-attention tasks performance (F = 0.29; p > 0.05; η2 = 0.001) revealed no significant condition effects (data not shown).
Cortisol awakening response (CAR)
The statistical analyses of the salivary cortisol levels revealed no significant main effect (p > 0.05). Nevertheless, post hoc testing revealed significantly increased cortisol levels at 08:00 (60 min post-awakening) in the caffeine condition when compared to placebo condition (t = 3.00; p < 0.04; Fig. 5).
Sleep characteristics
Nocturnal sleep
Given that the duration of wakefulness was experimentally prolonged prior to the initiation of nocturnal sleep, in both conditions, participants showed a sleep onset latency shorter than 10 min and more than 2 h of deep stage N3 sleep. For all sleep variables analyzed (Table 1), the linear mixed-effects models revealed no significant main effect of “condition” (F = 0.56; p > 0.05; η2 = 0.0025). Nevertheless, post hoc testing with the ‘R’ package emmeans indicated that the time spent in N3 sleep was shorter (p = 0.004) in the caffeine condition when compared to the placebo condition (Fig. 6A; Table 1).
Visually-sored sleep variables in (A) the 4-h nighttime sleep episode (03:00–07:00); (B) the final 10 min before scheduled awakening (06:50–07:00); and (C) the 1-h nap sleep opportunity (08:00–09:00) are shown. SOL, sleep onset latency; N1-3, non-rapid-eye-movement sleep stages N1-3; REM, rapid-eye movement sleep; *p < 0.05; **p < 0.01 (Benjamini–Hochberg corrected).
Pre-awakening sleep
Statistical analyses of sleep variables during 10 min before scheduled awakening revealed no significant main effect of ‘condition’ (F = 0.03; p > 0.05 η2 = 0.0001) (Fig. 6B; Table 1).
Nap sleep opportunity
The statistical analyses of the sleep variables in the nap opportunity 1 h after scheduled awakening revealed a significant ‘condition’ effect (F = 2.09; p < 0.01; η2 = 0.87), such that sleep onset latency and wakefulness after sleep onset were prolonged. In addition, the time spent in stage N2 was reduced in the caffeine condition when compared to the placebo condition (pall < 0.01; Fig. 6C; Table 1).
Discussion
Here we tested the in vivo drug release profile and efficacy to ameliorate morning sleep inertia of a delayed pulsatile-release caffeine formulation administered at bedtime. We found that this innovative approach potently facilitated the sleep-to-wake transition on neurobehavioral, subjective and physiological markers of sleep inertia in healthy young men following sleep restriction. Most importantly, in contrast to reactive caffeine intake, the novel formula improved vigilance within the first 15 min and mood at 15 min after waking in comparison to placebo.
The in vivo validation study corroborated the intended release profile. As expected based on the in vitro development of the engineered caffeine micropellets, the caffeine curve followed a delayed-release profile, and an efficacious plasma concentration above ~ 5 μM was attained only after 7 h. Unexpectedly, the release profile of the identical formulation in the pharmacodynamic study was distinctly different from the validation study. Premature caffeine-release started already 3.5 h after drug administration and efficacious plasma levels were attained already 5 h after drug intake. We suggest that a premature burst of the formula was probably triggered by the meal served to the subjects 3.5 h after caffeine administration (this meal was not served in the validation study). This food intake may have promoted gastric movements that caused faster gastric emptying and provoked the break of the release-modifying polymeric coat due to the physical impact of the peristalsis. Further studies are needed to confirm this hypothesis. In addition, future research may also clarify whether the pharmacokinetics of the engineered caffeine formula systematically differs between sleep and wakefulness.
Despite the premature release of caffeine, the formula ameliorated sleep inertia on the subsequent morning following only 4 h of sleep. The quality of awakening was subjectively improved on behavioral, cognitive, emotional, and physical levels, as indicated on all subscales of the sleep inertia questionnaire. Even though the study participants were sleep restricted, many of them reported less difficulty to rise when compared to a habitual morning, particularly in the caffeine condition. The formulation also exhibited mood enhancing properties, as indicated by increased positive and reduced negative ratings on the PANAS. This finding may suggest increased activity of mood-relevant brain structures in the caffeine condition when compared to the placebo condition. Caffeine blocks adenosine A2A receptors in the nucleus accumbens50,51, a core region of the mesolimbic dopamine network that is essential for the generation of positive mood and reward52. We speculate that caffeine-induced dopamine release in the nucleus accumbens53 could contribute to the post-awakening mood enhancement when compared to placebo. Other possible mechanisms include increased preparatory attention for rewarding stimuli54 or direct interaction with sleep–wake regulatory pathways such as the circadian clock55. The mechanistic underpinnings of the benefit of the pulsatile caffeine-release formulation on subjective state will have to be clarified in future studies.
The arousal effect of caffeine relies on the competitive antagonism of central nervous system adenosine receptors that contribute to the regulation of sleep intensity and sleep need14,50. Sleep inertia, in particular upon sleep restriction, was previously proposed to reflect ‘adenosine left overs’ that were insufficiently removed during sleep1,56. Consistent with this view, the primary beneficial effects of the tested caffeine release formula were not restricted to subjective state but included improved vigilance as manifested by faster PVT reaction times when compared to placebo. This finding is important because after nocturnal sleep, no easy applicable proactive or efficacious reactive countermeasure to impaired neurobehavioral performance due to sleep inertia is currently available6. Previous work suggested reduced lapsing on the PVT immediately after waking from a 30-min coffee-nap ending at 04:0024 and from repeated 2-h sleep opportunities during prolonged sleep deprivation16. In the present study, we observed no changes in the number of PVT lapses. The differences in the experimental protocols, as well as the caffeine dosages and application forms may underlie the discrepancy. Furthermore, we found no improvement in cognitively more demanding tasks such as sustained selective attention and visual scanning speed (assessed with the d2-task) and working memory and executive functioning (n-back task). These tasks were administered > 45 min after waking. Rested baseline measurements would be necessary to determine whether performance on these tasks at the time of their administration was impaired by sleep inertia and could be improved with the intervention tested.
After caffeine, the cortisol level on the CAR was increased 1 h after wake-up when compared to placebo. This finding supports the hypothesis that caffeine stimulates cortisol secretion and augments the CAR upon waking. The wake-up-related cortisol secretion was previously suggested to reflect a hormonal wake-promoting signal20. Nevertheless, neither the peak cortisol concentration nor the area-under-the-curve were affected by caffeine, suggesting that a direct association between reduced sleep inertia within the first 15 min of waking and HPA-axis activity is rather unlikely. Recent studies in rats revealed that overexpression of adenosine A2A receptors may contribute to glucocorticoid receptor dysfunctions in aged animals and that caffeine may re-sensitize glucocorticoid receptors in the hypothalamus and restore HPA-axis function57. Although the observed effect on the CAR in our young healthy sample was subtle, it may be speculated that a delayed-release caffeine formulation may promote cortisol release and glucocorticoid receptor functioning in susceptible individuals58.
Due to the sustained-release profile of the engineered formula, the blood caffeine concentration remained within an efficacious dose range until 17.5 h after administration. Consistent with this pharmacokinetic profile, the increased wake time after sleep onset, the prolonged sleep latency and the reduced N2 sleep duration during the 1-h nap after awakening support the notion that sustained low-dose caffeine administration improved post-awakening vigilance16. The premature high concentration of caffeine during nocturnal sleep most likely also underlies the ~ 17-min reduction in deep N3 sleep observed during the main sleep episode. Such a reduction in deep sleep would hamper the applicability of this novel pulsatile-release caffeine formula. Ongoing research employing quantitative sleep EEG analyses as a function of caffeine levels during sleep, as well as follow-up sleep studies will determine whether sleep is also disturbed without intra-night food intake. With respect to the reduced sleep depth, it seems unlikely that the mitigated sleep inertia after waking depended on the reduced duration of N3 sleep because this sleep state did not differ between the conditions during the final 10 min before scheduled awakening.
Taken together, this proof-of-concept investigation demonstrates that a timed pulsatile-release caffeine system ingested at bedtime can potently attenuate neurobehavioral, subjective, emotional and physiological manifestations of morning sleep inertia in sleep-restricted healthy young men. These findings cannot be generalized because only a single dose, only healthy men, and individuals irrespective of their caffeine sensitivity were studied. Nevertheless, if future research supports these conclusions and further improves the drug-release profile of the engineered formula, time-controlled caffeine administration may be developed as an add-on therapy to mitigate impaired morning state and vigilance in people suffering from excessive sleep inertia, which is highly prevalent in on-call and shift work settings, as well as in patients with neurological, neuropsychiatric, and circadian-rhythm sleep–wake disorders.
References
Hilditch, C. J. & McHill, A. W. Sleep inertia: Current insights. Nat. Sci. Sleep. 11, 155–165 (2019).
Hofer-Tinguely, G. et al. Sleep intertia: Performance changes after sleep, rest and active waking. Cogn. Brain Res. 22, 323–331 (2005).
Jewett, M. E. et al. Time course of sleep inertia dissipation in human performance and alertness. J. Sleep Res. 8, 1–8 (1999).
Tassi, P. & Muzet, A. Sleep inertia. Sleep Med. Rev. 4, 341–353 (2000).
Amaral, O. et al. Sleep patterns and insomnia among portuguese adolescents: A cross-sectional study. Aten Primaria. 46(Suppl 5), 191–194 (2014).
Hilditch, C. J., Dorrian, J. & Banks, S. Time to wake up: Reactive countermeasures to sleep inertia. Ind. Health. 54, 528–541 (2016).
Ohayon, M. M., Priest, R. G., Zulley, J. & Smirne, S. The place of confusional arousals in sleep and mental disorders—Findings in a general population sample of 13,057 subjects. J. Nerv. Ment. Dis. 188, 340–348 (2000).
Borisenkov, M. F. et al. Sleep characteristics, chronotype and winter depression in 10–20-year-olds in northern European Russia. J. Sleep Res. 24, 288–295 (2015).
Trenkwalder, C. et al. Rotigotine effects on early morning motor function and sleep in Parkinson’s disease: A double-blind, randomized, placebo-controlled study (RECOVER). Movement Disord. 26, 90–99 (2011).
Trotti, L. M. & Bliwise, D. L. Treatment of the sleep disorders associated with Parkinson’s disease. Neurotherapeutics 11, 68–77 (2014).
Broughton, R. J. Sleep disorders—disorders of arousal. Science 159, 1070–1078 (1968).
Bruck, D. & Pisani, D. L. The effects of sleep inertia on decision-making performance. J. Sleep Res. 8, 95–103 (1999).
Vyazovskiy, V. V. et al. The Dynamics of cortical neuronal activity in the first minutes after spontaneous awakening in rats and mice. Sleep 37, 1337–1347 (2014).
Landolt, H. P. Sleep homeostasis: A role for adenosine in humans?. Biochem. Pharmacol. 75, 2070–2079 (2008).
Nehlig, A. Are we dependent upon coffee and caffeine? A review on human and animal data. Neurosci. Biobehav .Rev. 23, 563–576 (1999).
Van Dongen, H. P. et al. Caffeine eliminates psychomotor vigilance deficits from sleep inertia. Sleep 24, 813–819 (2001).
Newman, R. A., Kamimori, G. H., Wesensten, N. J., Picchioni, D. & Balkin, T. J. Caffeine gum minimizes sleep inertia. Percept. Motor Skill. 116, 280–293 (2013).
Gonzaga, L. A., Vanderlei, L. C. M., Gomes, R. L. & Valenti, V. E. Caffeine affects autonomic control of heart rate and blood pressure recovery after aerobic exercise in young adults: A crossover study. Sci. Rep. 7, 1–8 (2017).
Lin, A. S., Uhde, T. W., Slate, S. O. & McCann, U. D. Effects of intravenous caffeine administered to healthy males during sleep. Depress Anxiety. 5, 21–28 (1997).
Boehringer, A. et al. Neural correlates of the cortisol awakening response in humans. Neuropsychopharmacol. 40, 2278–2285 (2015).
Clow, A., Hucklebridge, F., Stalder, T., Evans, P. & Thorn, L. The cortisol awakening response: More than a measure of HPA axis function. Neurosci. Biobehav. Rev. 35, 97–103 (2010).
Kudielka, B. M., Federenko, I. S., Hellhammer, D. H. & Wust, S. Morningness and eveningness: The free cortisol rise after awakening in “early birds” and “night owls”. Biol. Psychol. 72, 141–146 (2006).
Bonati, M. et al. Caffeine disposition after oral doses. Clin. Pharmacol. Ther. 32, 98–106 (1982).
Centofanti, S. et al. A pilot study investigating the impact of a caffeine-nap on alertness during a simulated night shift. Chronobiol. Int. 37, 1469–1473 (2020).
Landolt, H. P., Dijk, D. J., Gaus, S. E. & Borbély, A. A. Caffeine reduces low-frequency delta activity in the human sleep EEG. Neuropsychopharmacol. 12, 229–238 (1995).
Ballesta, A., Innominato, P. F., Dallmann, R., Rand, D. A. & Levi, F. A. Systems chronotherapeutics. Pharmacol. Rev. 69, 161–199 (2017).
Mandal, A. S. et al. Drug delivery system based on chronobiology—A review. J. Control Release 147, 314–325 (2010).
Naeem, M. et al. Colon-targeted delivery of budesonide using dual pH- and time-dependent polymeric nanoparticles for colitis therapy. Drug Des. Dev. Ther. 9, 3789–3799 (2015).
Akhgari, A., Sadeghi, F. & Garekani, H. A. Combination of time-dependent and pH-dependent polymethacrylates as a single coating formulation for colonic delivery of indomethacin pellets. Int. J. Pharm. 320, 137–142 (2006).
Song, L. J. et al. Optimizing pH-sensitive and time-dependent polymer formula of colonic pH-responsive pellets to achieve precise drug release. Asian J. Pharm. Sci. 14, 413–422 (2019).
Yadav, D., Survase, S. & Kumar, N. Dual coating of swellable and rupturable polymers on Glipizide loaded MCC pellets for pulsatile delivery: Formulation design and in vitro evaluation. Int. J. Pharm. 419, 121–130 (2011).
Broesder, A. et al. pH-dependent ileocolonic drug delivery, part I: In vitro and clinical evaluation of novel systems. Drug Discov. Today. 25, 1362–1373 (2020).
Wahlgren, M., Axenstrand, M., Hakansson, A., Marefati, A. & Pedersen, B. L. In vitro methods to study colon release: State of the art and an outlook on new strategies for better in-vitro biorelevant release media. Pharmaceutics. 11, 95 (2019).
Dorrian, J., Rogers, N. L. & Dinges, D. F. Psychomotor vigilance performance: a neurocognitive assay sensitive to sleep loss. In Sleep deprivation: Clinical issues, pharmacology and sleep loss effects (ed. Kushida, C.) 39–70 (Marcel Dekker, Inc., 2005).
Watson, D., Clark, L. A. & Tellegen, A. Development and validation of brief measures of positive and negative affect—The Panas scales. J. Pers. Soc. Psychol. 54, 1063–1070 (1988).
Rétey, J. V. et al. A genetic variation in the adenosine A2A receptor gene (ADORA2A) contributes to individual sensitivity to caffeine effects on sleep. Clin. Pharmacol. Ther. 81, 692–698 (2007).
Kanady, J. C. & Harvey, A. G. Development and validation of the sleep inertia questionnaire (SIQ) and assessment of sleep inertia in analogue and clinical depression. Cogn. Ther. Res. 39, 601–612 (2015).
Kirchner, W. K. Age-differences in short-term retention of rapidly changing information. J. Exp. Psychol. 55, 352–358 (1958).
Brickenkamp, R. Test d2 Aufmerksamkeits-Belastungs-Test 9th edn. (Hogrefe, 2002).
Binz, T. M., Braun, U., Baumgartner, M. R. & Kraemer, T. Development of an LC-MS/MS method for the determination of endogenous cortisol in hair using C-13(3)-labeled cortisol as surrogate analyte. J. Chromatogr. B 1033, 65–72 (2016).
Holst, S. C. et al. Cerebral mGluR5 availability contributes to elevated sleep need and behavioral adjustment after sleep deprivation. Elife. 6, e28751 (2017).
Weigend, S. et al. Dynamic changes in cerebral and peripheral markers of glutamatergic signaling across the human sleep-wake cycle. Sleep. 42, 161 (2019).
Hubbard, J. et al. Rapid fast-delta decay following prolonged wakefulness marks a phase of wake-inertia in NREM sleep. Nat. Commun. 11, 1–6 (2020).
Dornbierer, D. A. et al. Neurophysiological signature of gamma-hydroxybutyrate augmented sleep in male healthy volunteers may reflect biomimetic sleep enhancement: a randomized controlled trial. Neuropsychopharmacol. 44, 1985–1993 (2019).
Jasper, H. H. Report on the committee on methods of clinical examination in electroencephalography. Electroencephalogr. Clin. Neurophysiol. 10, 370–375 (1958).
Berry, R. B. et al. AASM Scoring Manual Updates for 2017 (Version 2.4). J. Clin. Sleep Med. 13, 665–6 (2017).
Hochberg, Y. & Benjamini, Y. More powerful procedures for multiple significance testing. Stat. Med. 9, 811–818 (1990).
Beaumont, M. et al. Slow release caffeine and prolonged (64-h) continuous wakefulness: Effects on vigilance and cognitive performance. J Sleep Res. 10, 265–276 (2001).
Lagarde, D. et al. Slow-release caffeine: A new response to the effects of a limited sleep deprivation. Sleep 23, 651–661 (2000).
Lazarus, M., Yoo, O. & Landolt, H.-P. Adenosinergic control of sleep. In Principles and Practice of Sleep Medicine 7th edn (eds Kryger, M. H. et al.) (Elsevier, Amsterdam, 2021).
Lazarus, M. et al. Arousal effect of caffeine depends on adenosine A(2A) receptors in the shell of the nucleus accumbens. J. Neurosci. 31, 10067–10075 (2011).
Nestler, E. J. & Carlezon, W. A. The mesolimbic dopamine reward circuit in depression. Biol. Psychiat. 59, 1151–1159 (2006).
Solinas, M. et al. Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens. J. Neurosci. 22, 6321–6324 (2002).
van den Berg, B., de Jong, M., Woldorff, M. G. & Lorist, M. M. Caffeine boosts preparatory attention for reward-related stimulus information. J. Cogn. Neurosci. 33, 104–118 (2021).
Trautmann, C., Burek, D., Hubner, C. A., Girault, J. A. & Engmann, O. A regulatory pathway linking caffeine action, mood and the diurnal clock. Neuropharmacol. 172, 108133 (2020).
Radulovacki, M., Miletich, R. S. & Green, R. D. N6 (L-Phenylisopropyl)adenosine (L-PIA) increases slow-wave sleep (S2) and decreases wakefulness in rats. Brain Res. 246, 178–180 (1982).
Batalha, V. L. et al. The caffeine-binding adenosine A(2A) receptor induces age-like HPA-axis dysfunction by targeting glucocorticoid receptor function. Sci Rep. 6, 1–5 (2016).
Nader, N., Chrousos, G. P. & Kino, T. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. Faseb. J. 23, 1572–1583 (2009).
Acknowledgements
We thank Ms. Gina Elrod and Ms. Tamara Bopp for their help with the data acquisition and Dr. Benjamin Stucky for his statistical support. Furthermore, we want to acknowledge the support of Mr. Robin von Rotz for the development of the modified version of the sleep inertia questionnaire.
Funding
This study was supported by the Clinical Research Priority Program “Sleep & Health” of the University of Zurich.
Author information
Authors and Affiliations
Contributions
D.A.D. and H.P.L. designed the study. D.A.D., R.W. and L.S. collected the data. D.A.D., F.Y., R.W., M.I.B., C.D.V., A.A., D.M.B., M.R.B., T.M.B. and H.P.L. analyzed and interpreted the data. F.Y., T.K. and H.P.L. provided resources, and experimental and analytical tools. D.A.D. and H.P.L. wrote the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dornbierer, D.A., Yerlikaya, F., Wespi, R. et al. A novel bedtime pulsatile-release caffeine formula ameliorates sleep inertia symptoms immediately upon awakening. Sci Rep 11, 19734 (2021). https://doi.org/10.1038/s41598-021-98376-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-98376-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.