COVID-19: World Health Organization advises against drug Donald Trump took to treat coronavirus
Health Secretary Matt Hancock also praised remdesivir when it was approved for use in the UK back in May.
Friday 20 November 2020 10:27, UK
The World Health Organization (WHO) is advising against the use of a drug Donald Trump took while suffering with coronavirus.
In May, Health Secretary Matt Hancock called the approval of the medicine for NHS patients the "biggest step forward in the treatment of coronavirus since the crisis began".
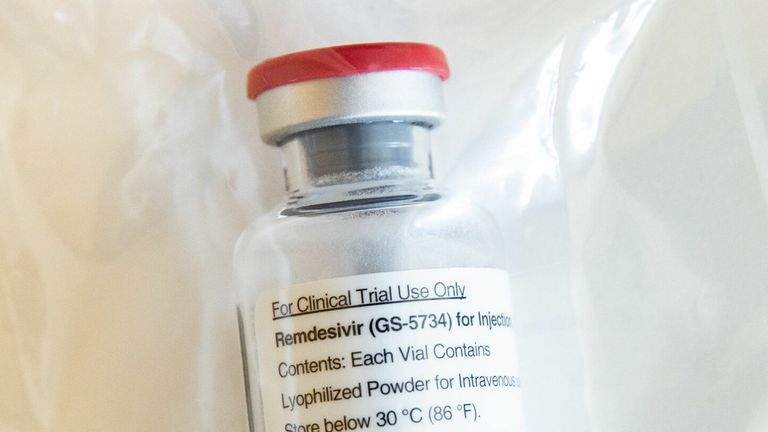
Remdesivir - also known as Veklury - is an antiviral therapeutic made by Gilead, and was originally intended to be used as an Ebola treatment.
However, new guidelines published by the WHO in The British Medical Journal found "a lack of evidence that remdesivir improved outcomes that matter to patients such as reduced mortality, need for mechanical ventilation, time to clinical improvement, and others".
Live COVID-19 updates from the UK and around the world
The WHO said in a statement: "The antiviral drug remdesivir is not suggested for patients admitted to hospital with COVID-19, regardless of how severely ill they are, because there is currently no evidence that it improves survival or the need for ventilation."
It was one of a cocktail of treatments given to President Trump when he was diagnosed with COVID-19 in the run up to the US election.
In the UK, it was given the green light for emergency use in May under the Early Access to Medicines Scheme.
Some trials initially showed that remdesivir could shorten hospital stays by four or five days.
But the WHO-lead "solidarity trial" showed that remdesivir had little or no effect on 28-day mortality or the length of time spent in hospital.
It is one of two medicines authorised at the moment to treat COVID-19 worldwide.
Epidemiology Professor Martin Landray from the University of Oxford, said: "As I and others have said before, we need scalable, affordable, and equitable treatments.
"The trials reported to date have shown no impact of remdesivir on survival."
He added: "Remember too that this is a drug that has to be given by intravenous infusion for five to 10 days and costs around £2,000 per course.
"So remdesivir is not cheap, it is not convenient, and it has no impact on the mortality among the people at highest risk."
However, manufacturer Gilead has questioned the WHO advice.
A statement said: "Veklury is recognised as a standard of care for the treatment of hospitalised patients with COVID-19 in guidelines from numerous credible national organisations.
"We are disappointed the WHO guidelines appear to ignore this evidence at a time when cases are dramatically increasing around the world and doctors are relying on Veklury as the first and only approved antiviral treatment for patients with COVID-19."
The Guideline Development Group at the WHO said it had made the announcement following a 7,000-person study from four international, randomised trials.
The WHO's advice is not binding, but rather part of the organisation's "living guidelines" project - this offers ongoing advice to doctors in fast-moving situations like the current pandemic.