Abstract
Several studies using cryogenic electron microscopy (cryo-EM) techniques recently reported the isolation and characterization of novel protein filaments, composed of a C-terminal fragment (CTF) of the endolysosomal transmembrane protein 106B (TMEM106B), from human post-mortem brain tissue with various neurodegenerative conditions and normal aging. Genetic variation in TMEM106B is known to influence the risk and presentation of several neurodegenerative diseases, especially frontotemporal dementia (FTD) caused by mutations in the progranulin gene (GRN). To further elucidate the significance of TMEM106B CTF, we performed immunohistochemistry with antibodies directed against epitopes within the filament-forming C-terminal region of TMEM106B. Accumulation of TMEM106B C-terminal immunoreactive (TMEM-ir) material was a common finding in all the conditions evaluated, including frontotemporal lobar degeneration with TDP-43 pathology (FTLD-TDP), Alzheimer’s disease, tauopathies, synucleinopathies and neurologically normal aging. TMEM-ir material was present in a wide range of brain cell types and in a broad neuroanatomical distribution; however, there was no co-localization of TMEM-ir material with other neurodegenerative proteins in cellular inclusions. In most conditions, the presence and abundance of TMEM-ir aggregates correlated strongly with patient age and showed only a weak correlation with the TMEM106B haplotype or the primary pathological diagnosis. However, all patients with FTD caused by GRN mutations were found to have high levels of TMEM-ir material, including several who were relatively young (< 60 years). These findings suggest that the accumulation of TMEM106B CTF is a common age-related phenomenon, which may reflect lysosomal dysfunction. Although its significance in most neurodegenerative conditions remains uncertain, the consistent finding of extensive TMEM-ir material in cases of FTLD-TDP with GRN mutations further supports a pathomechanistic role of TMEM106B and lysosomal dysfunction in this specific disease population.









Similar content being viewed by others
Data availability
All study data has been provided in paper and supplementary materials.
Change history
06 January 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00401-022-02536-y
References
Adams HH, Verhaaren BF, Vrooman HA, Uitterlinden AG, Hofman A, van Duijn CM et al (2014) TMEM106B influences volume of left-sided temporal lobe and interhemispheric structures in the general population. Biol Psychiatry 76:503–508. https://doi.org/10.1016/j.biopsych.2014.03.006
Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C et al (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442:916–919. https://doi.org/10.1038/nature05016
Brady OA, Zhou X, Hu F (2014) Regulated intramembrane proteolysis of the frontotemporal lobar degeneration risk factor, TMEM106B, by signal peptide peptidase-like 2a (SPPL2a). J Biol Chem 289:19670–19680. https://doi.org/10.1074/jbc.M113.515700
Busch JI, Martinez-Lage M, Ashbridge E, Grossman M, Van Deerlin VM, Hu F et al (2013) Expression of TMEM106B, the frontotemporal lobar degeneration-associated protein, in normal and diseased human brain. Acta Neuropathol Commun 1:36. https://doi.org/10.1186/2051-5960-1-36
Chang A, Xiang X, Wang J, Lee C, Arakhamia T, Simjanoska M et al (2022) Homotypic fibrillization of TMEM106B across diverse neurodegenerative diseases. Cell 185:1346-1355.e15. https://doi.org/10.1016/j.cell.2022.02.026
Chen-Plotkin AS, Unger TL, Gallagher MD, Bill E, Kwong LK, Volpicelli-Daley L et al (2012) TMEM106B, the risk gene for frontotemporal dementia, is regulated by the microRNA-132/212 cluster and affects progranulin pathways. J Neurosci 32:11213–11227. https://doi.org/10.1523/JNEUROSCI.0521-12.2012
DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ et al (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron 72:245–256. https://doi.org/10.1016/j.neuron.2011.09.011
Dugan AJ, Nelson PT, Katsumata Y, Shade LMP, Boehme KL, Teylan MA et al (2021) Analysis of genes (TMEM106B, GRN, ABCC9, KCNMB2, and APOE) implicated in risk for LATE-NC and hippocampal sclerosis provides pathogenetic insights: a retrospective genetic association study. Acta Neuropathol Commun 9:152. https://doi.org/10.1186/s40478-021-01250-2
Feng T, Mai S, Roscoe JM, Sheng RR, Ullah M, Zhang J et al (2020) Loss of TMEM106B and PGRN leads to severe lysosomal abnormalities and neurodegeneration in mice. EMBO Rep 21:e50219. https://doi.org/10.15252/embr.202050219
Feng T, Sheng RR, Solé-Domènech S, Ullah M, Zhou X, Mendoza CS et al (2020) A role of the frontotemporal lobar degeneration risk factor TMEM106B in myelination. Brain 143:2255–2271. https://doi.org/10.1093/brain/awaa154
Feng T, Lacrampe A, Hu F (2021) Physiological and pathological functions of TMEM106B: a gene associated with brain aging and multiple brain disorders. Acta Neuropathol 141:327–339. https://doi.org/10.1007/s00401-020-02246-3
Feng T, Luan L, Katz II, Ullah M, Van Deerlin VM, Trojanowski JQ et al (2022) TMEM106B deficiency impairs cerebellar myelination and synaptic integrity with Purkinje cell loss. Acta Neuropathol Commun 10:33. https://doi.org/10.1186/s40478-022-01334-7
Finch N, Carrasquillo MM, Baker M, Rutherford NJ, Coppola G, Dejesus-Hernandez M et al (2011) TMEM106B regulates progranulin levels and the penetrance of FTLD in GRN mutation carriers. Neurology 76:467–474. https://doi.org/10.1212/WNL.0b013e31820a0e3b
Gallagher MD, Suh E, Grossman M, Elman L, McCluskey L, Van Swieten JC et al (2014) TMEM106B is a genetic modifier of frontotemporal lobar degeneration with C9orf72 hexanucleotide repeat expansions. Acta Neuropathol 127:407–418. https://doi.org/10.1007/s00401-013-1239-x
Gallagher MD, Posavi M, Huang P, Unger TL, Berlyand Y, Gruenewald AL et al (2017) A dementia-associated risk variant near TMEM106B alters chromatin architecture and gene expression. Am J Hum Genet 101:643–663. https://doi.org/10.1016/j.ajhg.2017.09.004
Harding SR, Bocchetta M, Gordon E, Cash DM, Cardoso MJ, Druyeh R et al (2017) The TMEM106B risk allele is associated with lower cortical volumes in a clinically diagnosed frontotemporal dementia cohort. J Neurol Neurosurg Psychiatry 88:997–998. https://doi.org/10.1136/jnnp-2017-315641
Jiang YX, Cao Q, Sawaya MR, Abskharon R, Ge P, DeTure M et al (2022) Amyloid fibrils in FTLD-TDP are composed of TMEM106B and not TDP-43. Nature 605:304–309. https://doi.org/10.1038/s41586-022-04670-9
Lang CM, Fellerer K, Schwenk BM, Kuhn PH, Kremmer E, Edbauer D et al (2012) Membrane orientation and subcellular localization of transmembrane protein 106B (TMEM106B), a major risk factor for frontotemporal lobar degeneration. J Biol Chem 287:19355–19365. https://doi.org/10.1074/jbc.M112.365098
Li Z, Farias FHG, Dube U, Del-Aguila JL, Mihindukulasuriya KA, Fernandez MV et al (2020) The TMEM106B FTLD-protective variant, rs1990621, is also associated with increased neuronal proportion. Acta Neuropathol 139:45–61. https://doi.org/10.1007/s00401-019-02066-0
Llibre-Guerra JJ, Lee SE, Suemoto CK, Ehrenberg AJ, Kovacs GG, Karydas A et al (2021) A novel temporal-predominant neuro-astroglial tauopathy associated with TMEM106B gene polymorphism in FTLD/ALS-TDP. Brain Pathol 31:267–282. https://doi.org/10.1111/bpa.12924
Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E et al (2011) A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 122:111–113. https://doi.org/10.1007/s00401-011-0845-8
Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW et al (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123:1–11. https://doi.org/10.1007/s00401-011-0910-3
Nelson PT, Wang W-X, Partch AB, Monsell SE, Valladares O, Ellingson SR et al (2015) Reassessment of risk genotypes (GRN, TMEM106B, and ABCC9 variants) associated with hippocampal sclerosis of aging pathology. J Neuropathol Exp Neurol 74:75–84
Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y et al (2009) Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol 117:137–149. https://doi.org/10.1007/s00401-008-0477-9
Nicholson AM, Rademakers R (2016) What we know about TMEM106B in neurodegeneration. Acta Neuropathol 132:639–651. https://doi.org/10.1007/s00401-016-1610-9
Nicholson AM, Finch NA, Wojtas A, Baker MC, Perkerson RB 3rd, Castanedes-Casey M et al (2013) TMEM106B p. T185S regulates TMEM106B protein levels: implications for frontotemporal dementia. J Neurochem 126:781–791. https://doi.org/10.1111/jnc.12329
Perneel J, Rademakers R (2022) Identification of TMEM106B amyloid fibrils provides an updated view of TMEM106B biology in health and disease. Acta Neuropathol 144:807–819. https://doi.org/10.1007/s00401-022-02486-5
Rademakers R, Eriksen JL, Baker M, Robinson T, Ahmed Z, Lincoln SJ et al (2008) Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet 17:3631–3642. https://doi.org/10.1093/hmg/ddn257
Rademakers R, Nicholson AM, Ren Y, Koga S, Nguyen HP, Brooks M et al (2021) Loss of Tmem106b leads to cerebellum Purkinje cell death and motor deficits. Brain Pathol 31:e12945. https://doi.org/10.1111/bpa.12945
Rhinn H, Abeliovich A (2017) Differential aging analysis in human cerebral cortex identifies variants in TMEM106B and GRN that regulate aging phenotypes. Cell Syst 4:404-415.e5. https://doi.org/10.1016/j.cels.2017.02.009
Rutherford NJ, Carrasquillo MM, Li M, Bisceglio G, Menke J, Josephs KA et al (2012) TMEM106B risk variant is implicated in the pathologic presentation of Alzheimer disease. Neurology 79:717–718. https://doi.org/10.1212/WNL.0b013e318264e3ac
Satoh J, Kino Y, Kawana N, Yamamoto Y, Ishida T, Saito Y et al (2014) TMEM106B expression is reduced in Alzheimer’s disease brains. Alzheimers Res Ther 6:17. https://doi.org/10.1186/alzrt247
Schnell SA, Staines WA, Wessendorf MW (1999) Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem 47:719–730. https://doi.org/10.1177/002215549904700601
Schweighauser M, Arseni D, Bacioglu M, Huang M, Lövestam S, Shi Y et al (2022) Age-dependent formation of TMEM106B amyloid filaments in human brains. Nature 605:310–314. https://doi.org/10.1038/s41586-022-04650-z
Simons C, Dyment D, Bent SJ, Crawford J, D’Hooghe M, Kohlschütter A et al (2017) A recurrent de novo mutation in TMEM106B causes hypomyelinating leukodystrophy. Brain 140:3105–3111. https://doi.org/10.1093/brain/awx314
Stroobants S, D’Hooge R, Damme M (2021) Aged Tmem106b knockout mice display gait deficits in coincidence with Purkinje cell loss and only limited signs of non-motor dysfunction. Brain Pathol 31:223–238. https://doi.org/10.1111/bpa.12903
Tropea TF, Mak J, Guo MH, Xie SX, Suh E, Rick J et al (2019) TMEM106B Effect on cognition in Parkinson disease and frontotemporal dementia. Ann Neurol 85:801–811. https://doi.org/10.1002/ana.25486
van Blitterswijk M, Mullen B, Nicholson AM, Bieniek KF, Heckman MG, Baker MC et al (2014) TMEM106B protects C9ORF72 expansion carriers against frontotemporal dementia. Acta Neuropathol 127:397–406. https://doi.org/10.1007/s00401-013-1240-4
van Deerlin VM, Sleiman PM, Martinez-Lage M, Chen-Plotkin A, Wang LS, Graff-Radford NR et al (2010) Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet 42:234–239. https://doi.org/10.1038/ng.536
Vass R, Ashbridge E, Geser F, Hu WT, Grossman M, Clay-Falcone D et al (2011) Risk genotypes at TMEM106B are associated with cognitive impairment in amyotrophic lateral sclerosis. Acta Neuropathol 121:373–380. https://doi.org/10.1007/s00401-010-0782-y
Zhou X, Nicholson AM, Ren Y, Brooks M, Jiang P, Zuberi A et al (2020) Loss of TMEM106B leads to myelination deficits: implications for frontotemporal dementia treatment strategies. Brain 143:1905–1919. https://doi.org/10.1093/brain/awaa141
Acknowledgements
This work was funded by grants from the Canadian Institutes of Health Research (74580) and the National Institutes of Health (UAG063911) (IRAM); the University of Antwerp Research Funds (BOF), Vlaams Instituut voor Biotechnologie (VIB), and the National Institutes of Health (UG3 NS103870) (RR). J.P. is supported by a fellowship from Research Foundation—Flanders (FWO). J.F. is supported by a Holloway Postdoctoral fellowship from the Association for Frontotemporal Degeneration (AFTD). We would like to thank Manuel Gödan for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Rademakers is a member of the Scientific Advisory Board of Arkuda Therapeutics and receives invention royalties from a patent related to progranulin. Dr. Mackenzie is a member of the Scientific Advisory Board of Prevail Therapeutics and receives invention royalties from a patent related to progranulin.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article has been revised. Table 3 and 4 formatting are corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
401_2022_2531_MOESM2_ESM.tiff
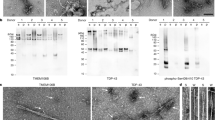
Supplementary file2 SB0051 recognizes TMEM106B C-terminal fragments but not full-length TMEM106B. Immunoblot was performed on samples from two representative cases (case 106 and 108) under conditions optimized for the detection of TMEM106B C-terminal fragments (CTF) (~30 kDa) (a) and for the detection of full-length TMEM106B (~43 kDa) (b, c). SB0051 detected CTFs in the sarkosyl-insoluble fraction only (a) and did not detected full-length TMEM106B in any of the fractions, even when conditions were optimized for the detection of the full-length protein (b). Full-length TMEM106B was detected in all fractions using an antibody that recognizes an N-terminal epitope of TMEM106B (c). S1 and S3, soluble fractions; P3, sarkosyl-insoluble fraction (TIFF 2171 KB)
401_2022_2531_MOESM3_ESM.tif
Supplementary file3 Comparison of TMEM106B C-terminal antibodies. The novel antiserum (SB0051) (a) and two commercially available antibodies raised against peptides corresponding to regions within the filament forming C-terminal fragment of TMEM106B (LSBio LS-C757550 (b) and Sigma SAB2106773 (c)) each demonstrated intracellular accumulation of material in sections of frontal cortex using standard IHC techniques in cases previously shown to have TMEM106B (120-254) filaments by cryo-EM [5]. Frontal cortex of case 7. Scale bar, 40 µm (TIF 7351 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Perneel, J., Neumann, M., Heeman, B. et al. Accumulation of TMEM106B C-terminal fragments in neurodegenerative disease and aging. Acta Neuropathol 145, 285–302 (2023). https://doi.org/10.1007/s00401-022-02531-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-022-02531-3