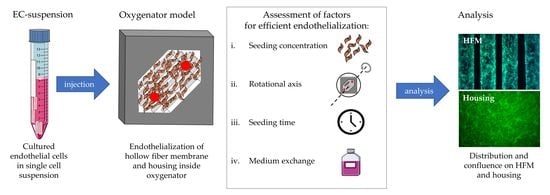
Biohybrid lung Development: Towards Complete Endothelialization of an Assembled Extracorporeal Membrane Oxygenator
Abstract
:1. Introduction
2. Materials and Methods
2.1. Endothelial Cell Culture and HFM Sample Preparation
2.1.1. Isolation, Cultivation and Characterization of hCBECs
2.1.2. HFM Fibronectin Coating for Reliable Endothelialization
2.2. Application of Standardized In Vitro Protocol for HFM Endothelialization for Model Oxygenator Assembly
2.2.1. Standardized In Vitro Endothelialization Protocol for Single-Layer HFMs Suitable for MOx Size
2.2.2. Transfer of In Vitro Reendothelialized HFMs for MOx Assembly
2.3. Establishment of the Optimal Endothelialization Protocol for Assembled MOx, Consisting of HFMs and Housing
2.3.1. Identifying the Optimal EC Concentration for Complete MOx Endothelialization
2.3.2. Identifying the Optimal MOx Rotational Axis for Optimal Seeding Procedure
2.3.3. Identifying the Ideal Rotation Time for Optimal Seeding Procedure
2.3.4. Identifying the Optimal Cell Culture Conditions during Seeding Procedure
Analysis of Culture Medium Conditions within Different Seeding Procedures
Impact Analysis of Cell Culture Medium Exchange during Seeding Procedure
2.4. Statistical Analysis
3. Results
3.1. Air Contact during Transfer of In Vitro Endothelialized HFMs for MOx Assembly Resulted in Significant Cell Damage
3.2. Succesful Protocol Establishment for Complete Endothelialization inside the Fully Assembled MOx
3.2.1. Eight-Fold Cell Concentration Resulted in Best MOx Endothelialization
3.2.2. Transversal Rotation Axis Improved EC Distribution in MOx
3.2.3. Prolonged Rotational Seeding under RA2 Enhanced EC Attachment in the MOx
3.2.4. Physiological Cell Medium Conditions were Required for Sufficient MOx Endothelialization
Prolonged Seeding Procedure was Associated with Significant Medium Consumption, Necessitating Periodic Medium Exchange within 24 h
Medium Exchange during 24 h Seeding Procedure Alleviated Apoptosis
Medium Substitution during 24 h Seeding Procedure Did Not Affect EML Integrity in Confluent Areas
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BHL | biohybrid lung |
| ECMO | extracorporeal membrane oxygenation |
| EC | endothelial cell |
| HFM | hollow fiber membrane |
| MOx | model oxygenator |
| LTx | lung transplantation |
| ELD | end-stage lung disease |
| EML | endothelial monolayer |
| PMP | poly-4-methyl-1-pentene |
| hCBEC | human cord blood endothelial cell |
| MNCs | mononuclear cells |
| EGM-2 | endothelial growth medium |
| FBS | fetal bovine serum |
| EDTA | ethylenediamine tetra-acetic acid |
| FN | fibronectin |
| SC | seeding concentration |
| RA | rotational axis |
| BGA | blood gas analyzer |
| MExch | medium exchange |
| PI | popidium iodide |
| TCP | tissue culture plastic |
| RT | room temperature |
| PFA | paraformaldehyde |
| BSA | bovine serum albumin |
| SD | standard deviation |
References
- ISHLT: The International Society for Heart & Lung Transplantation—TTX Registry Slides. Available online: https://ishlt.org/research-data/registries/ttx-registry/ttx-registry-slides (accessed on 24 August 2022).
- Heart. Available online: https://srtr.transplant.hrsa.gov/annual_reports/2020/Lung.aspx (accessed on 23 August 2022).
- Smits, J.M.; Nossent, G.; Evrard, P.; Lang, G.; Knoop, C.; Erp, J.M.K.-V.; Langer, F.; Schramm, R.; van de Graaf, E.; Vos, R.; et al. Lung allocation score: The Eurotransplant model versus the revised US model—A cross-sectional study. Transpl. Int. 2018, 31, 930–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottlieb, J. Lung allocation. J. Thorac. Dis. 2017, 9, 2670–2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The to10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 23 August 2022).
- Tonna, J.E.M.; Abrams, D.; Brodie, D.; Greenwood, J.C.; Mateo-Sidron, J.A.R.; Usman, A.M.; Fan, E.M. Management of Adult Patients Supported with Venovenous Extracorporeal Membrane Oxygenation (VV ECMO): Guideline from the Extracorporeal Life Support Organization (ELSO). ASAIO J. 2021, 67, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Biran, R.; Pond, D. Heparin coatings for improving blood compatibility of medical devices. Adv. Drug Deliv. Rev. 2017, 112, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Lehle, K.; Philipp, A.; Gleich, O.; Holzamer, A.; Müller, T.; Bein, T.; Schmid, C. Efficiency in Extracorporeal Membrane Oxygenation—Cellular Deposits on Polymethypentene Membranes Increase Resistance to Blood Flow and Reduce Gas Exchange Capacity. ASAIO J. 2008, 54, 612–617. [Google Scholar] [CrossRef]
- Callaghan, S.; Cai, T.; McCafferty, C.; Helm, S.V.D.; Horton, S.; MacLaren, G.; Monagle, P.; Ignjatovic, V. Adsorption of Blood Components to Extracorporeal Membrane Oxygenation (ECMO) Surfaces in Humans: A Systematic Review. J. Clin. Med. 2020, 9, 3272. [Google Scholar] [CrossRef]
- Rapid Activation of the Alternative Pathway of Complement by: ASAIO Journal. Available online: https://journals.lww.com/asaiojournal/Abstract/1999/01000/Rapid_Activation_of_the_Alternative_Pathway_of.25.aspx (accessed on 14 December 2022).
- Zimpfer, D.; Fiane, A.E.; Larbalestier, R.; Tsui, S.; Jansz, P.; Simon, A.; Schueler, S.; Strueber, M.; Schmitto, J.D. Long-Term Survival of Patients With Advanced Heart Failure Receiving an Left Ventricular Assist Device Intended as a Bridge to Transplantation. Circ. Hear. Fail. 2020, 13, e006252. [Google Scholar] [CrossRef]
- McGuigan, A.P.; Sefton, M.V. The influence of biomaterials on endothelial cell thrombogenicity. Biomaterials 2007, 28, 2547–2571. [Google Scholar] [CrossRef] [Green Version]
- Matsuzaki, Y.; John, K.; Shoji, T.; Shinoka, T. The Evolution of Tissue Engineered Vascular Graft Technologies: From Preclinical Trials to Advancing Patient Care. Appl. Sci. 2019, 9, 1274. [Google Scholar] [CrossRef] [Green Version]
- Koenneker, S.; Teebken, O.; Bonehie, M.; Pflaum, M.; Jockenhoevel, S.; Haverich, A.; Wilhelmi, M. A Biological Alternative to Alloplastic Grafts in Dialysis Therapy: Evaluation of an Autologised Bioartificial Haemodialysis Shunt Vessel in a Sheep Model. Eur. J. Vasc. Endovasc. Surg. 2010, 40, 810–816. [Google Scholar] [CrossRef]
- Dietrich, M.; Finocchiaro, N.; Olszweski, S.; Arens, J.; Schmitz-Rode, T.; Sachweh, J.; Jockenhoevel, S.; Cornelissen, C.G. ENDOXY—Development of a Biomimetic Oxygenator-Test-Device. PLoS ONE 2015, 10, e0142961. [Google Scholar] [CrossRef]
- Polk, A.A.; Maul, T.M.; McKeel, D.T.; Snyder, T.A.; Lehocky, C.A.; Pitt, B.; Stolz, D.B.; Federspiel, W.J.; Wagner, W.R. A biohybrid artificial lung prototype with active mixing of endothelialized microporous hollow fibers. Biotechnol. Bioeng. 2010, 106, 490–500. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/bit.22675 (accessed on 13 December 2022). [CrossRef] [Green Version]
- Klein, S.; Hesselmann, F.; Djeljadini, S.; Berger, T.; Thiebes, A.L.; Schmitz-Rode, T.; Jockenhoevel, S.; Cornelissen, C.G. EndOxy: Dynamic Long-Term Evaluation of Endothelialized Gas Exchange Membranes for a Biohybrid Lung. Ann. Biomed. Eng. 2019, 48, 747–756. [Google Scholar] [CrossRef] [Green Version]
- Pflaum, M.; Kühn-Kauffeldt, M.; Schmeckebier, S.; Dipresa, D.; Chauhan, K.; Wiegmann, B.; Haug, R.J.; Schein, J.; Haverich, A.; Korossis, S. Endothelialization and characterization of titanium dioxide-coated gas-exchange membranes for application in the bioartificial lung. Acta Biomater. 2017, 50, 510–521. [Google Scholar] [CrossRef]
- Takagi, M.; Shiwaku, K.; Inoue, T.; Shirakawa, Y.; Sawa, Y.; Matsuda, H.; Yoshida, T. Hydrodynamically stable adhesion of endothelial cells onto a polypropylene hollow fiber membrane by modification with adhesive protein. J. Artif. Organs 2003, 6, 222–226. [Google Scholar] [CrossRef]
- Zwirner, U.; Höffler, K.; Pflaum, M.; Korossis, S.; Haverich, A.; Wiegmann, B. Identifying an optimal seeding protocol and endothelial cell substrate for biohybrid lung development. J. Tissue Eng. Regen. Med. 2018, 12, 2319–2330. [Google Scholar] [CrossRef]
- Möller, L.; Hess, C.; Paleček, J.; Su, Y.; Haverich, A.; Kirschning, A.; Dräger, G. Towards a biocompatible artificial lung: Covalent functionalization of poly(4-methylpent-1-ene) (TPX) with cRGD pentapeptide. Beilstein J. Org. Chem. 2013, 9, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Pflaum, M.; Jurmann, S.; Katsirntaki, K.; Mälzer, M.; Haverich, A.; Wiegmann, B. Towards Biohybrid Lung Development—Fibronectin-Coating Bestows Hemocompatibility of Gas Exchange Hollow Fiber Membranes by Improving Flow-Resistant Endothelialization. Membranes 2022, 12, 35. [Google Scholar] [CrossRef]
- Hess, C.; Wiegmann, B.; Maurer, A.N.; Fischer, P.; Möller, L.; Martin, U.; Hilfiker, A.; Haverich, A.; Fischer, S. Reduced Thrombocyte Adhesion to Endothelialized Poly 4-Methyl-1-Pentene Gas Exchange Membranes—A First Step Toward Bioartificial Lung Development. Tissue Eng. Part A 2010, 16, 3043–3053. [Google Scholar] [CrossRef]
- Pflaum, M.; Dahlmann, J.; Engels, L.; Naghilouy-Hidaji, H.; Adam, D.; Zöllner, J.; Otto, A.; Schmeckebier, S.; Martin, U.; Haverich, A.; et al. Towards Biohybrid Lung: Induced Pluripotent Stem Cell Derived Endothelial Cells as Clinically Relevant Cell Source for Biologization. Micromachines 2021, 12, 981. [Google Scholar] [CrossRef]
- Wiegmann, B.; Figueiredo, C.; Gras, C.; Pflaum, M.; Schmeckebier, S.; Korossis, S.; Haverich, A.; Blasczyk, R. Prevention of rejection of allogeneic endothelial cells in a biohybrid lung by silencing HLA-class I expression. Biomaterials 2014, 35, 8123–8133. [Google Scholar] [CrossRef] [PubMed]
- Schlör, S.; Pflaum, M.; Höffler, K.; Kühn, C.; Haverich, A.; Wiegmann, B. Towards Biohybrid Lung Development: Establishment of a Porcine In Vitro Model. Membranes 2022, 12, 687. [Google Scholar] [CrossRef] [PubMed]
- Hesselmann, F.; Focke, J.M.; Schlanstein, P.C.; Steuer, N.B.; Kaesler, A.; Reinartz, S.D.; Schmitz-Rode, T.; Steinseifer, U.; Jansen, S.V.; Arens, J. Introducing 3D-potting: A novel production process for artificial membrane lungs with superior blood flow design. Bio. Des. Manuf. 2021, 5, 141–152. [Google Scholar] [CrossRef]
- Hess, C.; Schwenke, A.; Wagener, P.; Franzka, S.; Sajti, C.L.; Pflaum, M.; Wiegmann, B.; Haverich, A.; Barcikowski, S. Dose-dependent surface endothelialization and biocompatibility of polyurethane noble metal nanocomposites. J. Biomed. Mater. Res. Part A 2013, 102, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Wiegmann, B.; von Seggern, H.; Höffler, K.; Korossis, S.; Dipresa, D.; Pflaum, M.; Schmeckebier, S.; Seume, J.; Haverich, A. Developing a biohybrid lung—Sufficient endothelialization of poly-4-methly-1-pentene gas exchange hollow-fiber membranes. J. Mech. Behav. Biomed. Mater. 2016, 60, 301–311. [Google Scholar] [CrossRef]
- Ress, K.L.; Koerbin, G.; Li, L.; Chesher, D.; Bwititi, P.; Horvath, A.R. Reference intervals for venous blood gas measurement in adults. Clin. Chem. Lab. Med. CCLM 2020, 59, 947–954. [Google Scholar] [CrossRef]
- iLA MEMBRANE Ventilator | Xenios AG. Available online: https://www.xenios-ag.com/novalung/products/ila-membrane-ventilator/ (accessed on 14 December 2022).
- Quadrox-i Oxygenators. Available online: https://www.getinge.com/int/products/quadrox-i-oxygenators/ (accessed on 14 December 2022).
- Sobolewski, P.; Kandel, J.; Klinger, A.L.; Eckmann, D.M. Air bubble contact with endothelial cells in vitro induces calcium influx and IP3-dependent release of calcium stores. Am. J. Physiol. Physiol. 2011, 301, C679–C686. [Google Scholar] [CrossRef] [Green Version]
- Properties of Polysulfones. Available online: https://polymerdatabase.com/polymer%classes/Polysulfone%type.html (accessed on 14 December 2022).
- The Best Polymers for Medical Equipment Housings | Solvay. Available online: https://www.solvay.com/en/chemical-categories/specialty-polymers/healthcare/best-polymers-for-medical-equipment-housings (accessed on 14 December 2022).
- Burmeister, J.S.; Vrany, J.D.; Reichert, W.M.; Truskey, G.A. Effect of fibronectin amount and conformation on the strength of endothelial cell adhesion to HEMA/EMA copolymers. J. Biomed. Mater. Res. 1996, 30, 13–22. [Google Scholar] [CrossRef]
- Cornelissen, C.G.; Dietrich, M.; Gromann, K.; Frese, J.; Krueger, S.; Sachweh, J.A.; Jockenhoevel, S. Fibronectin Coating of Oxygenator Membranes Enhances Endothelial Cell Attachment Equal Contributors. 2013. Available online: http://www.biomedical-engineering-online.com/content/12/1/7 (accessed on 7 December 2021).
- Budd, J.S.; Allen, K.E.; Bell, P.R.; James, R.F. The effect of varying fibronectin concentration on the attachment of endothelial cells to polytetrafluoroethylene vascular grafts. J. Vasc. Surg. 1990, 12, 126–130. [Google Scholar] [CrossRef] [Green Version]
- Parra-Bonilla, G.; Alvarez, D.F.; Al-Mehdi, A.-B.; Alexeyev, M.; Stevens, T. Critical role for lactate dehydrogenase A in aerobic glycolysis that sustains pulmonary microvascular endothelial cell proliferation. Am. J. Physiol. Cell. Mol. Physiol. 2010, 299, L513–L522. [Google Scholar] [CrossRef]
- Eelen, G.; De Zeeuw, P.; Treps, L.; Harjes, U.; Wong, B.; Carmeliet, P. Endothelial Cell Metabolism. Physiol. Rev. 2018, 98, 3–58. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, S.M.; Gajdusek, C.M. Contact inhibition in the endothelium. In Biology of Endothelial Cells; Springer: New York, NY, USA, 1984; pp. 66–73. [Google Scholar] [CrossRef]
- Thornberry, N.A.; Lazebnik, Y. Caspases: Enemies within. Science 1998, 281, 1312–1316. [Google Scholar] [CrossRef]
- Rogers, S.C.; Zhang, X.; Azhar, G.; Luo, S.; Wei, J.Y. Exposure to High or Low Glucose Levels Accelerates the Appearance of Markers of Endothelial Cell Senescence and Induces Dysregulation of Nitric Oxide Synthase. J. Gerontol. Ser. A 2013, 68, 1469–1481. [Google Scholar] [CrossRef] [Green Version]
- Gulbins, H.; Pritisanac, A.; Petzold, R.; Goldemund, A.; Doser, M.; Dauner, M.; Meiser, B.; Reichart, B.; Daebritz, S. A Low-Flow Adaptation Phase Improves Shear-Stress Resistance of Artificially Seeded Endothelial Cells. Thorac. Cardiovasc. Surg. 2005, 53, 96–102. [Google Scholar] [CrossRef]
- Bein, T.; Weber, F.; Philipp, A.; Prasser, C.; Pfeifer, M.; Schmid, F.-X.; Butz, B.; Birnbaum, D.; Taeger, K.; Schlitt, H.J. A new pumpless extracorporeal interventional lung assist in critical hypoxemia/hypercapnia*. Crit. Care Med. 2006, 34, 1372–1377. [Google Scholar] [CrossRef] [Green Version]
- Yamane, T.; Mitsumata, M.; Yamaguchi, N.; Nakazawa, T.; Mochizuki, K.; Kondo, T.; Kawasaki, T.; Murata, S.-I.; Yoshida, Y.; Katoh, R. Laminar high shear stress up-regulates type IV collagen synthesis and down-regulates MMP-2 secretion in endothelium. A quantitative analysis. Cell Tissue Res. 2010, 340, 471–479. [Google Scholar] [CrossRef]







| pH (7.30–7.43) | Lactate (0.4–2.2 mmol/L) | Glucose (3.1–5.5 mmol/L) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Time [h] | |||||||||
| Seeding Condtionss | T0 | T6 | T24 | T0 | T6 | T24 | T0 | T6 | T24 |
| 6 h, RA1 | 7.40 ± 0.01 | 7.13 ± 0.02 | _ | 0.57 ± 0.05 | 2.90 ± 0.08 | _ | 5.37 ± 0.05 | 4.37 ± 0.05 | _ |
| 6 h, RA2 | 7.43 ± 0.06 | 7.02 ± 0.02 | _ | 0.50 ± 0.08 | 3.83 ± 0.05 | _ | 5.30 ± 0.00 | 4.30 ± 0.08 | _ |
| 24 h, RA1 | 7.36 ± 0.04 | _ | 6.78 ± 0.02 | 0.50 ± 0.00 | _ | 8.30 ± 0.14 | 5.30 ± 0.00 | _ | 1.97 ± 0.05 |
| 24 h, RA2 | 7.35 ± 0.01 | _ | 6.71 ± 0.02 | 0.40 ± 0.00 | _ | 8.57 ± 0.71 | 5.17 ± 0.05 | _ | 1.63 ± 0.26 |
| 24 h, RA2, MExch | 7.33 ± 0.01 | 7.03 ± 0.01 | 6.94 ± 0.05 | 0.57 ± 0.05 | 3.53 ± 0.24 | 3.47 ± 0.48 | 5.10 ± 0.00 | 4.07 ± 0.09 | 4.17 ± 0.12 |
| Oxygen (19–65 mmHg) | Carbon Dioxide (38–58 mmHg) | |||||
|---|---|---|---|---|---|---|
| Time [h] | ||||||
| Seeding Condtionss | T0 | T6 | T24 | T0 | T6 | T24 |
| 6 h, RA1 | 173 ± 0.00 | 136.67 ± 3.40 | _ | 17.77 ± 0.57 | 28.20 ± 1.27 | _ |
| 6 h, RA2 | 195 ± 2.94 | 147.33 ± 1.25 | _ | 17.50 ± 0.99 | 32.37 ± 1.56 | _ |
| 24 h, RA1 | 171 ± 1.41 | _ | 124.67 ± 9.46 | 19.47 ± 1.64 | _ | 37.77 ± 1.87 |
| 24 h, RA2 | 175 ± 0.82 | _ | 123.73 ± 20.98 | 18.83 ± 0.59 | _ | 38.90 ± 2.76 |
| 24 h, RA2, MExch | 177.67 ± 4.64 | 149 ± 5.35 | 140 ± 6.48 | 20 ± 0.71 | 32.80 ± 0.64 | 41.13 ± 3.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alabdullh, H.A.; Pflaum, M.; Mälzer, M.; Kipp, M.; Naghilouy-Hidaji, H.; Adam, D.; Kühn, C.; Natanov, R.; Niehaus, A.; Haverich, A.; et al. Biohybrid lung Development: Towards Complete Endothelialization of an Assembled Extracorporeal Membrane Oxygenator. Bioengineering 2023, 10, 72. https://doi.org/10.3390/bioengineering10010072
Alabdullh HA, Pflaum M, Mälzer M, Kipp M, Naghilouy-Hidaji H, Adam D, Kühn C, Natanov R, Niehaus A, Haverich A, et al. Biohybrid lung Development: Towards Complete Endothelialization of an Assembled Extracorporeal Membrane Oxygenator. Bioengineering. 2023; 10(1):72. https://doi.org/10.3390/bioengineering10010072
Chicago/Turabian StyleAlabdullh, Hussam Almesto, Michael Pflaum, Marisa Mälzer, Marcel Kipp, Hossein Naghilouy-Hidaji, Denise Adam, Christian Kühn, Russlan Natanov, Adelheid Niehaus, Axel Haverich, and et al. 2023. "Biohybrid lung Development: Towards Complete Endothelialization of an Assembled Extracorporeal Membrane Oxygenator" Bioengineering 10, no. 1: 72. https://doi.org/10.3390/bioengineering10010072