Abstract
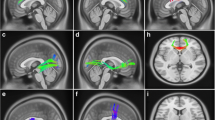
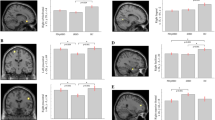
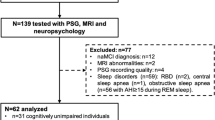
The influence of brain atrophy on sleep microstructure in Spinocerebellar Ataxias (SCAs) has not been extensively explored limiting the use of these sleep traits as surrogate biomarkers of neurodegeneration and clinical phenotype. The objective of the study is to explore the relationship between sleep microstructure and brain atrophy in SCA2 and its role in the clinical phenotype. Fourteen SCA2 mutation carriers (7 pre-manifest and 7 manifest subjects) underwent polysomnographic, structural MRI, and clinical assessments. Particularly, markers of REM and non-REM sleep microstructure, measures of cerebellar and brainstem atrophy, and clinical scores were analyzed through correlation and mediation analyses. The sleep spindle activity exhibited a negative correlation with the number of trials required to complete the verbal memory test (VMT), and a positive correlation with the cerebellar volume, but the significance of the latter correlation did not survive multiple testing corrections. However, the causal mediation analyses unveiled that sleep spindle activity significantly mediates the association between cerebellar atrophy and VMT performance. Regarding REM sleep, both phasic EMG activity and REM sleep without atonia exhibited significant associations with pontine atrophy and disease severity measures. However, they did not demonstrate a causal mediation effect between the atrophy measures and disease severity. Our study provides evidence about the association of the pontocerebellar atrophy with sleep microstructure in SCA2 offering insights into the cerebellar involvement in cognition via the control of the sleep spindle activity. Therefore, our findings may help to understand the disease pathogenesis and to better characterize sleep microstructure parameters as disease biomarkers.
Clinical trial registration number (TRN): No applicable.


Similar content being viewed by others
Data Availability
This dataset is not publicly available and can be asked from the Cuban authors directly upon reasonable request.
References
Velázquez-Pérez LC, Rodríguez-Labrada R, Fernandez-Ruiz J. Spinocerebellar ataxia type 2: clinicogenetic aspects, mechanistic insights, and management approaches. Front Neurol. 2017;8:472. Available from: https://journal.frontiersin.org/article/10.3389/fneur.2017.00472/full. Accessed 17 May 2023.
Tuin I, Voss U, Kang J-S, Kessler K, Rüb U, Nolte D, et al. Stages of sleep pathology in spinocerebellar ataxia type 2 (SCA2). Neurology. 2006;67:1966–72. Available from: https://www.neurology.org/lookup/doi/10.1212/01.wnl.0000247054.90322.14. Accessed 17 May 2023.
Boesch SM, Frauscher B, Brandauer E, Wenning GK, Högl B, Poewe W. Disturbance of rapid eye movement sleep in spinocerebellar ataxia type 2. Mov Disord. 2006;21:1751–4. Available from: https://movementdisorders.onlinelibrary.wiley.com/doi/10.1002/mds.21036. Accessed 17 May 2023.
Rodríguez-Labrada R, Velázquez-Perez L, Ochoa NC, Polo LG, Valencia RH, Cruz GS, et al. Subtle rapid eye movement sleep abnormalities in presymptomatic spinocerebellar ataxia type 2 gene carriers. Mov Disord. 2011;26:347–50. Available from: https://onlinelibrary.wiley.com/doi/10.1002/mds.23409. Accessed 17 May 2023.
Velázquez-Pérez L, Voss U, Rodríguez-Labrada R, Auburger G, Canales Ochoa N, Sánchez Cruz G, et al. Sleep disorders in spinocerebellar ataxia type 2 patients. Neurodegener Dis. 2011;8:447–54. Available from: https://www.karger.com/Article/FullText/324374. Accessed 17 May 2023.
Rodríguez-Labrada R, Galicia-Polo L, Canales-Ochoa N, Voss U, Tuin I, Peña-Acosta A, et al. Sleep spindles and K-complex activities are decreased in spinocerebellar ataxia type 2: relationship to memory and motor performances. Sleep Med. 2019;60:188–96. Available from: https://linkinghub.elsevier.com/retrieve/pii/S138994571930111X. Accessed 17 May 2023.
Selvadurai LP, Perlman SL, Wilmot GR, Subramony SH, Gomez CM, Ashizawa T, et al. The S-factor, a new measure of disease severity in spinocerebellar ataxia: findings and implications. The Cerebellum. 2022;22:790–809. Available from: https://link.springer.com/10.1007/s12311-022-01424-1. Accessed 27 Dec 2023.
Channon S, Daum I, Polkey CE. The effect of categorization on verbal memory after temporal lobectomy. Neuropsychologia. 1989;27:777–85. Available from: https://linkinghub.elsevier.com/retrieve/pii/002839328990002X. Accessed 18 Jun 2023.
Spreen O, Straus E. A compendium of neuropsychological tests: administration norms, and commentary. New York: Oxford University Press; 1991.
Spreen O, Benton A. Neurosensory center comprehensive examination for aphasia (NCCA). Victoria: University of Victoria, Neuropsychology Laboratory 1969.
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. Available from: https://linkinghub.elsevier.com/retrieve/pii/0165178189900474. Accessed 29 May 2023.
Johns MW. A new method for measuring daytime sleepiness: the epworth sleepiness scale. Sleep. 1991;14:540–5. Available from: http://academic.oup.com/sleep/article/14/6/540/2742871.
Berry RB, Brooks R, Gamaldo C, Harding SM, Lloyd RM, Quan SF, et al. AASM scoring manual updates for 2017 (Version 2.4). J Clin Sleep Med. 2017;13:665–6. Available from: http://jcsm.aasm.org/doi/10.5664/jcsm.6576.
Reetz K, Rodríguez-Labrada R, Dogan I, Mirzazade S, Romanzetti S, Schulz JB, et al. Brain atrophy measures in preclinical and manifest spinocerebellar ataxia type 2. Ann Clin Transl Neurol. 2018;5:128–37.
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–28. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1053811906000632. Accessed 17 May 2023.
Melpignano A, Parrino L, Santamaria J, Gaig C, Trippi I, Serradell M, et al. Isolated rapid eye movement sleep behavior disorder and cyclic alternating pattern: Is sleep microstructure a predictive parameter of neurodegeneration? Sleep. 2019;42:1–7.
Neylan TC, Walsh CM. Sleep spindles, tau, and neurodegeneration. Sleep 2022;45. Available from: https://academic.oup.com/sleep/article/doi/10.1093/sleep/zsac161/6633544.
Nigri A, Sarro L, Mongelli A, Pinardi C, Porcu L, Castaldo A, et al. Progression of cerebellar atrophy in spinocerebellar ataxia type 2 gene carriers: a longitudinal MRI study in preclinical and early disease stages. Front Neurol. 2020;11:1–12. Available from: https://www.frontiersin.org/articles/10.3389/fneur.2020.616419/full. Accessed 17 May 2023.
Koscik TR, Sloat L, van der Plas E, Joers JM, Deelchand DK, Lenglet C, et al. Brainstem and striatal volume changes are detectable in under 1 year and predict motor decline in spinocerebellar ataxia type 1. Brain Commun. 2020;2. Available from: https://academic.oup.com/braincomms/article/doi/10.1093/braincomms/fcaa184/6035119.
Schmahmann JD, Guell X, Stoodley CJ, Halko MA. The theory and neuroscience of cerebellar cognition. Annu Rev Neurosci. 2019;42:337–64.
Palesi F, De Rinaldis A, Castellazzi G, Calamante F, Muhlert N, Chard D, et al. Contralateral cortico-ponto-cerebellar pathways reconstruction in humans in vivo: implications for reciprocal cerebro-cerebellar structural connectivity in motor and non-motor areas. Sci Rep. 2017;7:12841. Available from: https://www.nature.com/articles/s41598-017-13079-8. Accessed 21 Dec 2023.
Hernandez‐Castillo CR, Galvez V, Mercadillo RE, Díaz R, Yescas P, Martinez L, et al. Functional connectivity changes related to cognitive and motor performance in spinocerebellar ataxia type 2. Mov Disord. 2015;30:1391–9. Available from: https://movementdisorders.onlinelibrary.wiley.com/doi/10.1002/mds.26320. Accessed 20 Dec 2023.
Olivito G, Cercignani M, Lupo M, Iacobacci C, Clausi S, Romano S, et al. Neural substrates of motor and cognitive dysfunctions in SCA2 patients: a network based statistics analysis. NeuroImage Clin. 2017;14:719–25. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2213158217300694. Accessed 21 Dec 2023.
Okayasu M, Inukai T, Tanaka D, Tsumura K, Shintaki R, Takeda M, et al. The Stroop effect involves an excitatory–inhibitory fronto-cerebellar loop. Nat Commun. 2023;14:27. Available from: https://www.nature.com/articles/s41467-022-35397-w. Accessed 18 Dec 2023.
Jung BC, Choi SI, Du AX, Cuzzocreo JL, Ying HS, Landman BA, et al. MRI shows a region-specific pattern of atrophy in spinocerebellar ataxia type 2. Cerebellum. 2012;11:272–9.
Hoche F, Guell X, Vangel MG, Sherman JC, Schmahmann JD. The cerebellar cognitive affective / Schmahmann syndrome scale. Brain. 2017;141:248–70.
Lindsay E, Storey E. Cognitive changes in the spinocerebellar ataxias due to expanded polyglutamine tracts: a survey of the literature. Brain Sci. 2017;7:83. Available from: https://www.mdpi.com/2076-3425/7/7/83. Accessed 21 Dec 2023.
Almeida-Silva UC, Hallak JEC, Júnior WM, de Lima OF. Association between spinocerebellar ataxias caused by glutamine expansion and psychiatric and neuropsychological signals - A literature review. Am J Neurodegener Dis. 2013;2:57–69.
Arrigoni E, Chen MC, Fuller PM. The anatomical, cellular and synaptic basis of motor atonia during rapid eye movement sleep. J Physiol. 2016;19:5391–414.
St Louis EK, McCarter SJ, Boeve BF, Silber MH, Kantarci K, Benarroch EE, et al. Lesional REM sleep behavior disorder localizes to the dorsomedial pons. Neurology. 2014;83(20):1871–73.
Campabadal A, Segura B, Junque C, Iranzo A. Structural and functional magnetic resonance imaging in isolated REM sleep behavior disorder: A systematic review of studies using neuroimaging software. Sleep Med Rev. 2021;59:101495. https://doi.org/10.1016/j.smrv.2021.101495.
Heller J, Brcina N, Dogan I, Holtbernd F, Romanzetti S, Schulz JB, et al. Brain imaging findings in idiopathic REM sleep behavior disorder (RBD) – A systematic review on potential biomarkers for neurodegeneration. Sleep Med Rev. 2017;34:23–33.
Huebra L, Coelho FM, Moura F, Filho R, Barsottini OG. Sleep disorders in hereditary ataxias. Curr Neurol Neurosci Rep. 2019;19(8):59.
Schabus M, Dang-Vu TT, Albouy G, Balteau E, Boly M, Carrier J, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci U S A. 2007;104:13164–9.
Xu W, De Carvalho F, Clarke AK, Jackson A. Communication from the cerebellum to the neocortex during sleep spindles. Prog Neurobiol. 2021;199:101940. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0301008220301957. Accessed 31 May 2023.
Jackson A, Xu W. Role of cerebellum in sleep-dependent memory processes. Front Syst Neurosci. 2023;17:1–11.
Fernandez LMJ, Lüthi A. Sleep spindles: mechanisms and functions. Physiol Rev. 2020;100:805–68. Available from: https://journals.physiology.org/doi/10.1152/physrev.00042.2018. Accessed 29 May 2023.
Fang Z, Ray LB, Owen AM, Fogel SM. Brain activation time-locked to sleep spindles associated with human cognitive abilities. Front Neurosci. 2019;13:1–16.
Schönauer M, Pöhlchen D. Sleep spindles. Curr Biol. 2018;28:R1129–30. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0960982218309345. Accessed 31 May 2023.
Acknowledgements
The authors thank all SCA2 subjects for their cooperation. We also thank the Cuban Ministry of Public Health and the Federal Ministry of Education and Research of Germany (BMBF 01DN18022) for providing the funds used in the present study.
Funding
This work was supported by the Cuban Ministry of Public Health and the Federal Ministry of Education and Research of Germany (BMBF 01DN18022).
Author information
Authors and Affiliations
Contributions
RRL and LVP: Conceptualization and study design.
RRL, LVP and GA: Literature search.
RRL, NCO, ECR, APA, AER, YVM: Data collection.
RRL, MLGP, SR: Data analysis.
RRL, LVP, SR, ID, GA, KR: Data interpretation.
RRL: Writing-original draft.
LVP, SR, ID, KR and GA: Writing-review & editing.
Corresponding authors
Ethics declarations
Ethical Approval
The study was approved by the ethics committee of the Centre for the Research and Rehabilitation of Hereditary Ataxias (Holguin, Cuba), and was conducted according to the Declaration of Helsinki. Written informed consent was obtained from each participant.
Competing Interests
The authors declare no competing interests.
Financial Disclosure
All Cuban authors received funding from the Cuban Ministry of Public Health, whereas RRL, SR, ID, KR and LVP received funding from the Federal Ministry of Education and Research of Germany (BMBF 01DN18022). None has received any other funding for the last 12 months relevant or not for this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Luis Velázquez-Pérez is a senior author.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rodríguez-Labrada, R., Canales-Ochoa, N., Galicia-Polo, M.d.L. et al. Structural Brain Correlates of Sleep Microstructure in Spinocerebellar Ataxia Type 2 and its Role on Clinical Phenotype. Cerebellum (2024). https://doi.org/10.1007/s12311-024-01674-1
Accepted:
Published:
DOI: https://doi.org/10.1007/s12311-024-01674-1