Abstract
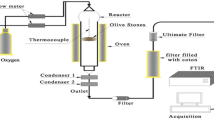
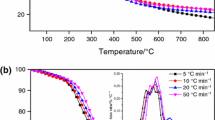
In the last decades, one of the most ordinary challenges that countries of Southern Europe face is the management of wastes and by-products of olive oil production activities which are disposed into the environment and contribute to the enhancement of major ecological issues due to their organic loads. Exploitation of these olive residues wastes by thermochemical treatment has been proved a very popular way to generate a wide range of valuable products. In this concept, this work studies the thermal decomposition process of olive stone samples using thermogravimetric analysis (TGA) data obtained under inert atmosphere at various heating rates (5–20 °C) and their kinetic analysis conducted via model-free and model fitting methods. The changes in the crystal structure for various pyrolysis temperatures of the sample were examined by X-ray diffraction analysis (XRD), while the morphological characteristics were examined by scanning electron microscopy (SEM). Results indicated that the thermal behavior of olive stone sample was a typical one for a lignocellulosic material with the first mass loss being attributed to moisture removal while the following stages were assigned to lignocellulosic degradation substances. Vyazovkin (VYA) isoconversional method was performed to estimate effective activation energy (Ea) and the pre-exponential factor, while using a model fitting method the best fitting results were obtained by a three independent parallel reactions model, obeying the nth order with Fn code and described by the f(α) = (1 − α)n equation.






Similar content being viewed by others
References
Koçer O, Acemioğlu B. Adsorption of Basic green 4 from aqueous solution by olive pomace and commercial activated carbon: process design, isotherm, kinetic and thermodynamic studies. Desalin Water Treat. 2016;57:16653–69.
Braadbaart F, Marinova E, Sarpaki A. Charred olive stones: experimental and archaeological evidence for recognizing olive processing residues used as fuel. Veg Hist Archaeobot. Springer Berlin Heidelberg 2016;25:415–30.
Dermeche S, Nadour M, Larroche C, Moulti-Mati F, Michaud P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem [Internet]. Elsevier Ltd 2013;48:1532–52. Available from https://doi.org/10.1016/j.procbio.2013.07.010.
Ghanem MTM, Tawfik WA, Mahdy E-SM, Abdelgawad ME, Abdel-Azim NS, El-Missiry MM. Chemical and biological evaluation of olive leaves as a waste by-product of olive oil industry. Egypt Pharm J. 2019;18:172–7.
Sánchez-Gutiérrez M, Espinosa E, Bascón-Villegas I, Pérez-Rodríguez F, Carrasco E, Rodríguez A. Production of cellulose nanofibers from olive tree harvest—a residue with wide applications. Agronomy. 2020;10:1–15.
Dardouri S, Jalila S. A comparative study of adsorption and regeneration with different agricultural wastes as adsorbents for the removal of methylene blue from aqueous solution. Chinese J Chem Eng [Internet]. Elsevier B.V. 2017;25:1282–7. Available from https://doi.org/10.1016/j.cjche.2017.01.012.
Ghouma I, Jeguirim M, Guizani C, Ouederni A, Limousy L. Pyrolysis of Olive Pomace: degradation kinetics, gaseous analysis and char characterization. Waste Biomass Valorization. Springer Netherlands 2017;8:1689–97.
Ceylan S. Kinetic analysis on the non-isothermal degradation of plum stone waste by thermogravimetric analysis and integral Master-Plots method. Waste Manag Res. 2015;33:345–52.
Guida MY, Bouaik H, El Mouden M, Moubarik A, Aboulkas A, El harfi K, Hannioui A. Utilization of Starink Approach and Avrami Theory to evaluate the kinetic parameters of the pyrolysis of olive mill solid waste and olive mill wastewater. J Adv Chem Eng. 2017;07:1–8.
Hu X, Gholizadeh M. Biomass pyrolysis: a review of the process development and challenges from initial researches up to the commercialisation stage. J Energy Chem [Internet]. Elsevier B.V. and Science Press 2019;39:109–43. Available from https://doi.org/10.1016/j.jechem.2019.01.024.
Manić N, Janković B, Dodevski V. Model-free and model-based kinetic analysis of Poplar fluff (Populus alba) pyrolysis process under dynamic conditions. J Therm Anal Calorim [Internet]. Springer International Publishing; 2020; Available from https://doi.org/10.1007/s10973-020-09675-y.
Sobek S, Werle S. Kinetic modelling of waste wood devolatilization during pyrolysis based on thermogravimetric data and solar pyrolysis reactor performance. Fuel. Elsevier; 2020;261.
Guida MY, Bouaik H, Tabal A, Hannioui A, Solhy A, Barakat A, et al. Thermochemical treatment of olive mill solid waste and olive mill wastewater: Pyrolysis kinetics. J Therm Anal Calorim. Springer Netherlands 2016;123:1657–66.
Damartzis T, Vamvuka D, Sfakiotakis S, Zabaniotou A. Thermal degradation studies and kinetic modeling of cardoon (Cynara cardunculus) pyrolysis using thermogravimetric analysis (TGA). Bioresour Technol [Internet]. Elsevier Ltd 2011;102:6230–8. Available from https://doi.org/10.1016/j.biortech.2011.02.060.
Moukhina E. Determination of kinetic mechanisms for reactions measured with thermoanalytical instruments. J Therm Anal Calorim. 2012;109:1203–14.
Brachi P, Miccio F, Miccio M, Ruoppolo G. Isoconversional kinetic analysis of olive pomace decomposition under torrefaction operating conditions. Fuel Process Technol [Internet]. Elsevier B.V. 2015;130:147–54. Available from https://doi.org/10.1016/j.fuproc.2014.09.043.
Ounas A, Aboulkas A, El harfi K, Bacaoui A, Yaacoubi A. Pyrolysis of olive residue and sugar cane bagasse: Non-isothermal thermogravimetric kinetic analysis. Bioresour Technol [Internet]. Elsevier Ltd 2011;102:11234–8. Available from https://doi.org/10.1016/j.biortech.2011.09.010.
Gao Y, Yang Y, Qin Z, Sun Y. Factors affecting the yield of bio-oil from the pyrolysis of coconut shell. Springerplus. Springer International Publishing 2016;5.
Samolada MC, Stoicos T, Vasalos IA. An investigation of the factors controlling the pyrolysis product yield of Greek wood biomass in a fluidized bed. J Anal Appl Pyrolysis. 1990;18:127–41.
Prakash Bamboriya O, Singh Thakur L, Parmar H, Kumar Varma A, Hinge VK. A review on mechanism and factors affecting pyrolysis of biomass. Int J Res Advent Technol [Internet]. 2019;7:1014–24. Available from www.ijrat.org.
Leng L, Huang H. An overview of the effect of pyrolysis process parameters on biochar stability. Bioresour Technol [Internet]. Elsevier Ltd; 2018;270:627–42. Available from https://doi.org/10.1016/j.biortech.2018.09.030.
Vyazovkin S. Modification of the integral isoconversional method to account for variation in the activation energy. J Comput Chem. 2001;22:178–83.
Vyazovkin S. Evaluation of activation energy of thermally stimulated solid-state reactions under arbitrary variation of temperature. J Comput Chem. 1997;18:393–402.
Alshammari BA, Alotaibi MD, Alothman OY, Sanjay MR, Kian LK, Almutairi Z, et al. A new study on characterization and properties of natural fibers obtained from Olive Tree (Olea europaea L.) residues. J Polym Environ [Internet]. Springer US 2019;27:2334–40. Available from https://doi.org/10.1007/s10924-019-01526-8.
Elbir M, Moubarik A, Rakib EM, Grimi N, Amhoud A, Miguel G, et al. Valorization of moroccan olive stones by using it in particleboard panels. Maderas Cienc y Tecnol. 2012;14:361–71.
Gomez-Martin A, Chacartegui R, Ramirez-Rico J, Martinez-Fernandez J. Performance improvement in olive stone’s combustion from a previous carbonization transformation. Fuel [Internet]. Elsevier 2018;228:254–62. Available from https://doi.org/10.1016/j.fuel.2018.04.127.
Al-Farraji A, Marsh R, Steer J. A comparison of the pyrolysis of olive kernel biomass in fluidised and fixed bed conditions. Waste Biomass Valorization. Springer Netherlands 2017;8:1273–84.
Luz Yolanda Toro Suarez. No Biomass Gasification and Pyrolysi. 2015.
Gai C, Dong Y, Zhang T. The kinetic analysis of the pyrolysis of agricultural residue under non-isothermal conditions. Bioresour Technol [Internet]. Elsevier Ltd 2013;127:298–305. Available from https://doi.org/10.1016/j.biortech.2012.09.089.
Šimon P, Thomas P, Dubaj T, Cibulková Z, Peller A, Veverka M. The mathematical incorrectness of the integral isoconversional methods in case of variable activation energy and the consequences. J Therm Anal Calorim. 2014;115:853–9.
Chrissafis K. Kinetics of thermal degradation of polymers. J Therm Anal Calorim. 2009;95.
Sobek S, Werle S. Kinetic modelling of waste wood devolatilization during pyrolysis based on thermogravimetric data and solar pyrolysis reactor performance. Fuel [Internet]. Elsevier 2020;261:116459. Available from https://doi.org/10.1016/j.fuel.2019.116459.
Koutsomitopoulou AF, Bénézet JC, Bergeret A, Papanicolaou GC. Preparation and characterization of olive pit powder as a filler to PLA-matrix bio-composites. Powder Technol [Internet]. Elsevier B.V. 2014;255:10–6. Available from https://doi.org/10.1016/j.powtec.2013.10.047.
Khalideh Al bkoor Alrawashdeh, Katarzyna Slopiecka, Abdullah A. Alshorman, Pietro Bartocci, Francesco Fantozzi. Pyrolytic Degradation of Olive Waste Residue (OWR) by TGA: thermal decomposition behavior and kinetic study. J Energy Power Eng. 2017;11.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Acknowledgements
The author would like to thank Pharmagnose S.A. (Athens) for collaboration and supply of olive stone samples. Funding was provided by GSRI (Grant No. Project Code: T6YBΠ- 00161).
Funding
This research has been co-financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call Special Actions AQUACULTURE-INDUSTRIAL MATERIALS-OPEN INNOVATION IN CULTURE (project code: T6YBΠ- 00161).
Author information
Authors and Affiliations
Contributions
ΤΑ performed the experiments, material characterization measurements and evaluation. KC and TA wrote the paper. KC supervised the research study.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Asimakidou, T., Chrissafis, K. Thermal behavior and pyrolysis kinetics of olive stone residue. J Therm Anal Calorim 147, 9045–9054 (2022). https://doi.org/10.1007/s10973-021-11163-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-11163-w