Research on the Treatment and Comprehensive Utilization of Phenylhydrazine Hydrochloride Effluent
Abstract
:1. Introduction
2. Production Principle of PHH
2.1. Aniline Diazotization
2.2. Diazonium Salt Reduction
2.3. Acid Precipitation Analysis and Salt Formation
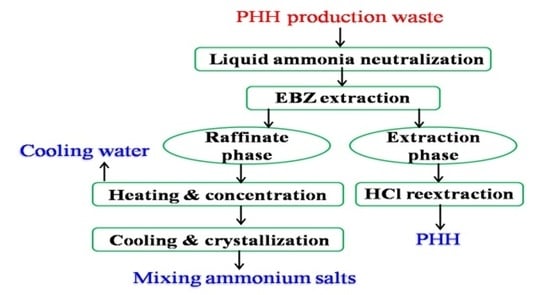
3. Treatment of PHH Production Waste
3.1. Liquid Ammonia Neutralization
3.2. Ethylbenzene Extraction
3.3. Hydrochloric Acid Reextraction
3.4. Raffinate Evaporation Concentration
3.5. Schematic Diagram of the Treatment Process of PHH Production Waste
4. Results and Discussion
4.1. Bench Scale Test
4.2. Pilot Scale Test
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zheng, J.; Huang, F.; Li, Y.; Xu, T.; Xu, H.; Jia, J.; Ye, Q.; Gao, J. The aggregation-induced emission enhancement properties of BF2 complex isatin-phenylhydrazone: Synthesis and fluorescence characteristics. Dyes Pigments 2015, 113, 502–509. [Google Scholar] [CrossRef]
- Banerjee, M.; Ray, A.K. The role of thyroid hormone on phenylhydrazine hydrochloride mediated inhibitory effects on blood acetylcholinesterase: An in vivo and in vitro study. J. Biochem. Mol. Toxicol. 2002, 16, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Ramyashree, D.; Raghavendra, K.R.; Kumar, A.D.; Vagish, C.B.; Kumar, K.A. Synthesis, characterization and antimicrobial activities of chalcones and their post transformation to pyrazole derivatives. Asian J. Chem. 2017, 29, 1538–1542. [Google Scholar] [CrossRef]
- Tao, X.; Wei, D.; Bao, X. Research on treatment of phenylhydrazine hydrochloride wastewater. Technol. Dev. Chem. Ind. 2013, 42, 39–41. [Google Scholar]
- Heinz, H. Process for the Production of Phenylhydrazine Hydrochloride. U.S. Patent 3,203,989, 31 August 1965. [Google Scholar]
- Zhang, R.; Wu, Y.; Zheng, D. The Synthesis of phenylhydrazine hydrochloride. J. Nanchang Univ. (Nat. Sci.) 2002, 26, 394–396. [Google Scholar]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent advancement of coagulation-flocculation and its application in wastewater treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Wu, T.Y.; Mohammad, A.W.; Lim, S.L.; Lim, P.N.; Hay, J.X.W. Recent advances in the reuse of wastewaters for promoting sustainable development. In Wastewater Reuse and Management; Sharma, S.K., Sanghi, R., Eds.; Springer: Dordrecht, The Netherlands, 2013; Volume 3, pp. 47–103. ISBN 9789400749412. [Google Scholar]
- Dufresne, C.; Leblanc, Y.; Berthelette, C.; McCooeye, C. The synthesis of phenylhydrazines from bis(2,2,2-Trichloroethyl) azodicarboxylates and electron-rich arenes. Synth. Commun. 1997, 27, 3613–3624. [Google Scholar] [CrossRef]
- Fu, C. Synthesis of high purity phenylhydrazine. Fine Spec. Chem. 2004, 12, 19–21. [Google Scholar]
- Li, J.; Sun, T.; Wang, Y. Research on the synthesis of 1,2-Diphenylhydrazine. Chem. World 2002, 43, 650–652. [Google Scholar]




| Material Name | Mass/kg |
|---|---|
| Aniline | 300.0 |
| NaNO2 | 232.0 |
| 30% HCl (diazotization reaction) | 835.0 |
| 56% NH4HSO3 | 1400.0 |
| 22% NH3·H2O | 180.0 |
| 30% HCl (acid precipitation analysis) | 870.0 |
| PHH (theoretical value) | 415 |
| PHH (actual output) | 370 |
| Material Name | Mass/kg |
|---|---|
| Total liquid waste | 3600 |
| Dissolved PHH | 45 |
| Consumed Liquid ammonia | 230 |
| Transformed PHZ | 33 |
| 30% HCl | 45 |
| Recycled PHH | 41 |
| Recycled ammonium salts | 1600 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhou, D.; Zhang, H. Research on the Treatment and Comprehensive Utilization of Phenylhydrazine Hydrochloride Effluent. Water 2018, 10, 438. https://doi.org/10.3390/w10040438
Liu X, Zhou D, Zhang H. Research on the Treatment and Comprehensive Utilization of Phenylhydrazine Hydrochloride Effluent. Water. 2018; 10(4):438. https://doi.org/10.3390/w10040438
Chicago/Turabian StyleLiu, Xide, Di Zhou, and Heng Zhang. 2018. "Research on the Treatment and Comprehensive Utilization of Phenylhydrazine Hydrochloride Effluent" Water 10, no. 4: 438. https://doi.org/10.3390/w10040438