Potential of Biochar Filters for Onsite Wastewater Treatment: Effects of Biochar Type, Physical Properties and Operating Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Characterisation of Material
2.3. Chemical Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Physical and Chemical Properties of Biochar and Sand
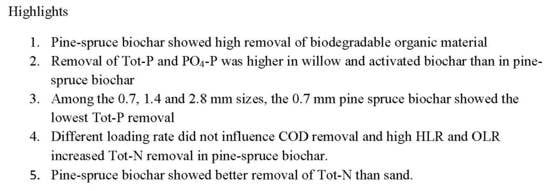
3.2. Treatment Performance
3.2.1. Influent Characteristics
3.2.2. Organic Matter Removal
3.2.3. Nitrogen
3.2.4. Phosphorus
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- SMED. Teknikenkät—Enskilda avlopp 2009; Ek, M., Junestedt, C., Larsson, C., Olshammar, M., Ericsson, M., Eds.; SMED: Norrköping, Sweden, 2011; p. 49. [Google Scholar]
- Hav- och vattenmyndigheten. Näringsbelastningen på Östersjön och Västerhavet 2014. In Sveriges underlag till Helcoms sjätte Pollution Load Compilation; Hav- och vattenmyndigheten: Göteborg, Sweden, 2016; p. 110. [Google Scholar]
- Lindberg, R.H.; Wennberg, P.; Johansson, M.I.; Tysklind, M.; Andersson, B.A. Screening of Human Antibiotic Substances and Determination of Weekly Mass Flows in Five Sewage Treatment Plants in Sweden. Environ. Sci. Technol. 2005, 10, 3421–3429. [Google Scholar] [CrossRef]
- Noyola, A.; Padilla-Rivera, A.; Morgan-Sagastume, J.M.; Güereca, L.P.; Hernández-Padilla, F. Typology of Municipal Wastewater Treatment Technologies in Latin America. Clean Soil Air Water 2012, 9, 926–932. [Google Scholar] [CrossRef]
- Cossio, C.; McConville, J.; Rauch, S.; Wilén, B.M.; Dalahmeh, S.; Mercado, A.; Romero, A.M. Wastewater management in small towns—Understanding the failure of small treatment plants in Bolivia. Environ. Technol. 2018, 39, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Spychała, M.; Błazejewski, R. Sand filter clogging by septic tank effluent. Water Sci. Technol. 2003, 48, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Steiner, C. Considerations in Biochar Characterization. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; Guo, M., He, Z., Uchimiya, S.M., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Ye, S.; Zeng, G.; Wu, H.; Zhang, C.; Dai, J.; Liang, J.; Yu, J.; Ren, X.; Yi, H.; Cheng, M.; et al. Biological technologies for the remediation of co-contaminated soil. Crit. Rev. Biotechnol. 2017, 8, 1062–1076. [Google Scholar] [CrossRef] [PubMed]
- Downie, A.; Krosky, A.; Munroe, P. Physical Properties of Biochar. In Biochar for Environmental Management Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009. [Google Scholar]
- Dalahmeh, S.S.; Lalander, C.; Pell, M.; Vinnerås, B.; Jönsson, H. Quality of greywater treated in biochar filter and risk assessment of gastroenteritis due to household exposure during maintenance and irrigation. J. Appl. Microbiol. 2016, 5, 1427–1443. [Google Scholar] [CrossRef]
- He, Z.; Uchimiya, S.M.; Guo, M. (Eds.) Production and Characterization of Biochar from Agricultural By-Products: Overview and Use of Cotton Biomass Residues. In Agricultural and Environmental Applications of Biochar: Advances and Barriers; Soil Science Society of America, Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Niwagaba, C.B.; Dinno, P.; Wamala, I.; Dalahmeh, S.S.; Lalander, C.; Jönsson, H. Experiences on the implementation of a pilot grey water treatment and reuse based system at a household in the slum of Kyebando-Kisalosalo, Kampala. J. Water Reuse Desalin. 2014, 4, 294–307. [Google Scholar] [CrossRef] [Green Version]
- Dalahmeh, S. Capacity of Biochar Filters for Wastewater Treatment in Onsite Systems—Technical Report; Report 2016-90; Havs och vattenmyndighet: Göteborg, Sweden; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2016; ISBN 978-91-576-9398-3.
- Dalahmeh, S.S.; Jönsson, H.; Hylander, L.D.; Hui, N.; Yu, D.; Pell, M. Dynamics and functions of bacterial communities in bark, charcoal and sand filters treating greyawter. Water Res. 2014, 54, 21–32. [Google Scholar] [CrossRef]
- Ye, S.; Zeng, G.; Wu, H.; Liang, J.; Zhang, C.; Dai, J.; Xiong, W.; Song, B.; Wu, S.; Yu, J. The effects of activated biochar addition on remediation efficiency of co-composting with contaminated wetland soil. Resources. Conserv. Recycl. 2019, 140, 278–285. [Google Scholar] [CrossRef]
- Rolland, L.; Molle, P.; Liénard, A.; Bouteldja, F.; Grasmick, A. Influence of the physical and mechanical characteristics of sands on the hydraulic and biological behaviors of sand filters. Desalination 2009, 248, 998–1007. [Google Scholar] [CrossRef]
- Beal, C.D.; Rassam, D.W.; Gardner, E.A.; Kirchhof, G.; Menzies, N.W. Influence of hydraulic loading and effluent flux on surface surcharging in soil absorption systems. J. Hydrol. Eng. 2008, 8, 681–692. [Google Scholar] [CrossRef]
- Dalahmeh, S.S.; Pell, M.; Hylander, L.D.; Lalander, C.; Vinnerås, B.; Jönsson, H. Effects of changing hydraulic and organic loading rates on pollutant reduction in bark, charcoal and sand filters treating greywater. J. Environ. Manag. 2014, 132, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Dane, J.H.; Topp, C.G. Methods of Soil Analysis, Part 4: Physical Methods; Soil Science Society of America, Inc.: Madison, WI, USA, 2002. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 2, 309–319. [Google Scholar] [CrossRef]
- Lens, P.N.; Vochten, P.M.; Speleers, L.; Verstraete, W.H. Direct treatment of domestic wastewater by percolation over peat, bark and woodchips. Water Res. 1994, 1, 17–26. [Google Scholar] [CrossRef]
- Korber, D.R.; Lawrence, J.R.; Lappin-Scott, H.M. Growth of Microorganisms on Surfaces. In Microbial Biofilms; Lappin-Scott, H.M., Costerton, J., Eds.; Cambridge University Press: Cambridge, UK, 2003; p. 15. [Google Scholar]
- Tabet, T.A.; Aziz, F.A. Cellulose Microfibril Angle in Wood and Its Dynamic Mechanical Significance. In Cellulose—Fundamental Aspects; Ven, T.v.d., Godbout, L., Eds.; InTech: Rijeka, Croatia, 2013; p. 05. [Google Scholar]
- Huggins, T.M.; Haeger, A.; Biffinger, J.C.; Ren, Z.J. Granular biochar compared with activated carbon for wastewater treatment and resource recovery. Water Res. 2016, 94, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Kholoma, E.; Renman, G.; Renman, A. Phosphorus removal from wastewater by fieldscale fortified filter beds during a one-year study. Environ. Technol. 2016, 23, 2953–2963. [Google Scholar] [CrossRef]
- Çeçen, F.; Aktaş, Ö. Integration of Activated Carbon Adsorption and Biological Processes in Wastewater Treatment. In Activated Carbon for Water and Wastewater Treatment: Integration of Adsorption and Biological Treatment; Wiley-VCH: Weinheim, Germany, 2011; pp. 43–93. [Google Scholar]
- Stevik, T.K.; Aa, K.; Ausland, G.; Hanssen, J.F. Retention and removal of pathogenic bacteria in wastewater percolating through porous media: A review. Water Res. 2004, 6, 1355–1367. [Google Scholar] [CrossRef]
- Halalsheh, M.; Dalahmeh, S.; Sayed, M.; Suleiman, W.; Shareef, M.; Mansour, M.; Safi, M. Grey water characteristics and treatment options for rural areas in Jordan. Bioresour. Technol. 2008, 99, 6635–6641. [Google Scholar] [CrossRef]
- Vinnerås, B.; Palmquist, H.; Balmér, P.; Jönsson, H. The characteristics of household wastewater and biodegradable solid waste—A proposal for new Swedish design values. Urban Water J. 2006, 1, 3–11. [Google Scholar] [CrossRef]
- Pell, M.; Nyberg, F. Infiltration of waste-water in a newly started pilot sand-filter system. 1. Reduction of organic-matter and phosphorus. J. Environ. Qual. 1989, 4, 451–457. [Google Scholar] [CrossRef]
- Wilson, J.; Boutilier, L.; Jamieson, R.; Havard, P.; Lake, C. Effects of Hydraulic Loading Rate and Filter Length on the Performance of Lateral Flow Sand Filters for On-Site Wastewater Treatment. J. Hydrol. Eng. 2011, 8, 639–649. [Google Scholar] [CrossRef]
- Wijeyekoon, S.; Mino, T.; Satoh, H.; Matsuo, T. Effects of substrate loading rate on biofilm structure. Water Res. 2004, 10, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Gill, L.W.; O’luanaigh, N.; Johnston, P.M.; Misstear, B.D.; O’suilleabhain, C. Nutrient loading on subsoils from on-site wastewater effluent, comparing septic tank and secondary treatment systems. Water Res. 2009, 10, 2739–2749. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.A.; Brix, H.; Johansen, N.H. Phosphorus removal from municipal wastewater in an experimental two-stage vertical flow constructed wetland system equipped with a calcite filter. Water Sci. Technol. 2003, 5, 51–58. [Google Scholar] [CrossRef]








| Specifications | Sub-Study 1 | Sub-Study 2 | Sub-Study 3 | Sub-Study 4 | Sub-Study 5 |
|---|---|---|---|---|---|
| Type of material | (1) Non-activated willow biochar (2) Non-activated pine-spruce biochar (3) Activated biochar | (1) Non-activated pine-spruce biochar | (1) Non-activated pine-spruce biochar | (1) Non-activated pine-spruce biochar | (1) Non-activated pine-spruce biochar (2) Sand |
| Number of replicates for each medium and treatment | 3 | 3 | 3 | 3 | 3 |
| Effective size (d10, mm) | 1.4 | 0.7, 1.4, and 2.8 | 1.4 | 1.4 | 1.4 |
| Hydraulic loading rate (L m−2 day−1) | 32 | 34 | 200 and 34 | 200 and 37 | 37 |
| Organic loading rate (g BOD5 m−2 day−1) | 15-20 | 20 | 5 and 20 | 5 | 5 |
| Chemical pollutants | COD, BOD5 NH4, NO3, Tot-N, PO4-P, Tot-P | COD, BOD7, NO3, Tot-N, PO4-P, Tot-P | COD, BOD7, NH4, NO3, Tot-N | COD, BOD7, NH4, NO3, Tot-N | COD, BOD7, NO3, Tot-N, PO4-P, Tot-P |
| Filter operation period (weeks) | 20 | 26 | 20 | 20 | 26 |
| Substance | Kit Name | Measurement Range | Units | Standard Method | Control Solution Name and Value | Apparatus | |
|---|---|---|---|---|---|---|---|
| EC | Electrical conductivity | mS cm−1 | Calibration liquid: KCl 500 µS/cm | Conductivity Pocket Meter, Cond340i WTW, Germany | |||
| pH | Standard Unit (SU) | Calibration liquid: pH 7 and pH 9 | pH-meter Ino Lab pH Level 1, WTW pH-electrode Blueline 14 pH, Schott instruments | ||||
| COD | Chemical oxygen demand | Spectroquant COD Cell Test (Hg-free) 1.09772.0001 and 1.09773.0001 | 10–150 and 100–1500 | mg L−1 | No standard, but Hg-free | Potassium hydrogen phthalate solution 1.11769.0100, Merck 170 mg L−1 and Combi R1, Combicheck 20 1.14675.0001, Merck 750 ± 75 mg L−1 | Thermoreactor TR 420, Merck, Germany Spectroquant NOVA 60, Merck, Germany Pipettor, VWR, Poland Analog Vortex Mixer, VWR, USA |
| NH4-N | Ammonium | Spectroquant Ammonium Cell Test 1.14544.0001 | 0.5–16 | mg L−1 | EPA 350.1, US Standard Methods 4500-NH3 D, and ISO 7150/1 | Combi R1, Combicheck 20 1.14675.0001, Merck 12 ± 1 mg L−1 | Spectroquant NOVA 60, Merck, Germany Pipettor*, VWR, Poland Analog Vortex Mixer, VWR, USA |
| NO3-N | Nitrate | Spectroquant Nitrate Cell Test 1.14764.0001 | 1–50 | mg L−1 | Nitrate standard solution 1.19811.0500, Merck 1000 mg L−1 | Spectroquant NOVA 60, Merck, Germany Pipettor*, VWR, Poland Analog Vortex Mixer, VWR, USA | |
| Tot-N | Total nitrogen | Spectroquant Nitrogen (total) Cell Test 1.147630001 and 1.00613.001 | 10–150 and 0.5–15 | mg L−1 | EN ISO 11905-1 (digestion) | Nitrate standard solution 1.19811.0500, Merck 1000 mg L−1 | Thermoreactor TR 420, Merck, Germany Spectroquant NOVA 60, Merck, Germany Pipettor*, VWR, PolandAnalog Vortex Mixer, VWR, USA |
| Tot-P | Total phosphorus | Spectroquant Phosphate Cell Test 1.14543.0001 | 0.05-5 | mg L−1 | EPA 365.2 + 3, APHA 4500-P E, and DIN EN ISO 6878 | Phosphate standard solution 1.19898.0500, Merck 1000 mg L−1 | Thermoreactor TR 420, Merck, Germany Spectroquant NOVA 60, Merck, Germany Pipettor*, VWR, Poland Analog Vortex Mixer, VWR, USA |
| PO4-P | Phosphate | Spectroquant Phosphate Cell Test 1.14543.0001 | 0.05-5 | mg L−1 | EPA 365.2+3, APHA 4500-P E, and DIN EN ISO 6878 | Phosphate standard solution 1.19898.0500, Merck 1000 mg L−1 | Spectroquant NOVA 60, Merck, Germany Pipettor*, VWR, Poland Analog Vortex Mixer, VWR, USA |
| Filter Material | Activated Biochar | Non-Activated Willow Biochar | Non-Activated Pine-Spruce Biochar | Sand |
|---|---|---|---|---|
| Particle size (mm) | 1.5 and 2.8–5 | 1–1.4 and 2.8–5 | 1.4–5 | 1.4–5 |
| Air-dry water content (%) | 0.6 | 6.3 | ||
| Specific surface area (m2/g) | >1000 | 170–200 | 0.152 | |
| Bulk density (kg m−3) | 560 | 270 | 187 | 1690 |
| Particle density (kg m−3) | 1890 | 740 | 2570 | |
| Total porosity (%) | 70.6 | 63.3 | 72–74 | 34 |
| Water-filled porosity (%) | 48–53 | |||
| Mean residence time (h) | 119 | 108 | 87 1; 85 2; 66 3 | 0.5 |
| Hydraulic conductivity (cm h−1) | 500 | 360 |
| Specifications | Sub-Study 1 | Sub-Study 2 | Sub-Study 3 | Sub-Study 4 | Sub-Study 5 | ||
|---|---|---|---|---|---|---|---|
| Type of material | (1) Non-activated willow biochar (2) Non-activated pine-spruce biochar (3) Activated biochar * | (1) Non-activated pine-spruce biochar | (1) Non-activated pine-spruce biochar | (1) Non-activated pine-spruce biochar | (1) Non-activated pine-spruce biochar (2) Sand | ||
| Effective size (d10, mm) | 1.4 | 0.7, 1.4, 2.8 | 1.4 | 1.4 | 1.4 | ||
| Hydraulic loading rate (L m−2 day−1) | 32 | 34 | 200 | 34 | 37 | 200 | 37 |
| Organic loading rate (g BOD5 m−2 day−1) | 15–20 | 20 | 5 | 20 | 5 | 5 | 5 |
| COD (mg L−1) | 1230–1140 | 1229 ± 320 | 325 ± 103 | 1229 ± 320 | 496 ± 87 | 325 ± 103 | 496 ± 87 |
| BOD7 (mg L−1) | 490–630 * | 629 ± 105 | 26 ± 10 | 629 ± 105 | 131 ± 50 | 26 ± 10 | 131 ± 50 |
| NO3-N | 1–3 | 1.3 ± 2.5 | 17 ± 8 | 1.3 ± 2.5 | 6 ± 6 | 17 ± 8 | 6 ± 6 |
| NH4-N | 3.7–11.0 | 11 ± 9 | 7 ± 3 | 11 ± 9 | 7 ± 3 | ||
| T-N (mg L−1) | 78–95 | 78 ± 27 | 26 ± 8 | 78 ± 27 | 30 ± 4 | 26 ± 8 | 30 ± 4 |
| PO4-P (mg L−1) | 2.6–3.2 | 3.2 ± 0.8 | 3.2 ± 0.8 | 1.87 ± 0.94 | 1.87 ± 0.94 | ||
| Tot-P (mg L−1) | 3.6–3.8 | 3.8 ± 0.7 | 3.8 ± 0.7 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Mercado, L.F.; Lalander, C.; Berger, C.; Dalahmeh, S.S. Potential of Biochar Filters for Onsite Wastewater Treatment: Effects of Biochar Type, Physical Properties and Operating Conditions. Water 2018, 10, 1835. https://doi.org/10.3390/w10121835
Perez-Mercado LF, Lalander C, Berger C, Dalahmeh SS. Potential of Biochar Filters for Onsite Wastewater Treatment: Effects of Biochar Type, Physical Properties and Operating Conditions. Water. 2018; 10(12):1835. https://doi.org/10.3390/w10121835
Chicago/Turabian StylePerez-Mercado, Luis Fernando, Cecilia Lalander, Christina Berger, and Sahar S. Dalahmeh. 2018. "Potential of Biochar Filters for Onsite Wastewater Treatment: Effects of Biochar Type, Physical Properties and Operating Conditions" Water 10, no. 12: 1835. https://doi.org/10.3390/w10121835