Treatment of Eutrophic Water and Wastewater from Valsequillo Reservoir, Puebla, Mexico by Means of Ozonation: A Multiparameter Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
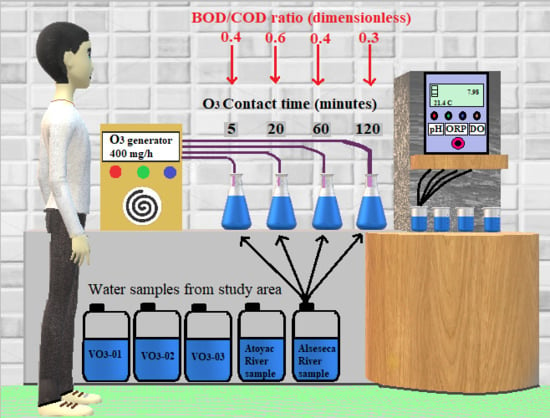
2.2. Experimental Design
2.3. Measurement of Physicochemical Parameters
2.4. Calculation of Kinetics
3. Results and Discussion
3.1. Raw Water Characteristics of the Different Studied Sites
3.2. Ozone Effect over Organic Matter (SAC254, COD and BOD5)
3.2.1. Organic Matter Removal (%) as Function of Different O3 Doses
3.2.2. Degradation Kinetics of COD, BOD5 and SAC254
3.3. Ozone Effect over Physicochemical Parameters
3.3.1. Organoleptic Properties
3.3.2. Total Phosphorous (TP)
3.3.3. Potential of Hydrogen (pH)
3.3.4. Conductivity
3.3.5. Ammonia Nitrogen (NH3-N) and Nitrates (NO3−)
3.3.6. Dissolved Oxygen (DO) and Redox-Potential (ORP)
3.4. Ozone Effect over Major and Trace Metals
3.5. Ozone Effect over Organic Compounds
3.6. Ozone Effect over Disinfection (Fecal Coliforms Removal)
4. Conclusions
- Water from the Alseseca River and the Atoyac River present characteristics of raw urban wastewaters, while VO3-02 and VO3-03 water samples display characteristics of secondary effluents. On the other hand, VO3-01 showed features of both raw and secondary effluents.
- Concerning the SAC254 levels, the results showed a decrease of the aromatic fraction in the organic molecules or unsaturated molecules through direct ozonation, as the SAC254 removal was found to be 31.7% in VO3-02 water sample. The maximum COD removal was 60.2% for VO3-AL water sample with a 0.26 mg O3/mg initial COD dose. Meanwhile, for BOD5, the maximum removal was observed to be 55% in VO3-AT water sample with a 4.6 mg O3/mg initial COD dose.
- A higher content of organic compounds (particularly recalcitrant compounds) was detected in the area of the Valsequillo Reservoir that is covered with Water hyacinths. This is presumably due to aquatic plant’s decomposition process when they are in their senescence stage.
- Results regarding the BOD5/COD ratio showed that the O3 treatment increased the biodegradability in the OM of all the water samples, improving the water quality and hence setting the conditions for the optimization of the natural attenuation process currently carried out in the Reservoir.
- Ozonation of heavy metals revealed that the process highly depends on the water’s alkalinity, since the presence of calcium determines whether the metals precipitate or remain in their dissolved form
- Among all the phthalates studied, DEHP showed the highest concentration levels (5.8 μg/L in the water sample VO3-AT). During ozonation of the VO3-01 water sample, an increase in the DEHP, DBP, DEP and DMP content was registered owing to the ozone reaction with recalcitrant organic compounds.
- Results concerning FC showed that the O3 treatment is only suitable for water with low OM concentrations, since OM acts as an O3 scavenger leading to a scarce contact between FC and ozone.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rice, R.G.; Netzer, A. Handbook of Ozone Technology and Applications; Ann Arbor Science: Ann Arbor, MI, USA, 1982. [Google Scholar]
- Jabesa, A.; Ghosh, P. Removal of dimethyl phthalate from water by ozone microbubbles. Environ. Technol. 2017, 38, 2093–2103. [Google Scholar] [CrossRef] [PubMed]
- Hoigne, J.; Bader, H. Ozonation of Water: Role of Hydroxyl Radicals as Oxidizing Intermediates. Science 1975, 190, 782–784. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marc-olivier, B.; Schumacher, J.; Sébastien, M.; Jekel, M.; Urs von, G. Ozonation and Advanced Oxidation of Wastewater: Effect of O3 Dose, pH, DOM and HO•-Scavengers on Ozone Decomposition and HO• Generation. Ozone Sci. Eng. 2006, 28, 247–259. [Google Scholar]
- Ratpukdi, T.; Siripattanakul, S.; Khan, E. Mineralization and biodegradability enhancement of natural organic matter by ozone-VUV in comparison with ozone, VUV, ozone-UV, and UV: Effects of pH and ozone dose. Water Res. 2010, 44, 3531–3543. [Google Scholar] [CrossRef] [PubMed]
- Thalmann, B.; von Gunten, U.; Kaegi, R. Ozonation of municipal wastewater effluent containing metal sulfides and metal complexes: Kinetics and mechanisms. Water Res. 2018, 134, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Grasso, D.; Weber, W.J. Ozone-Induced Particle Destabilization. J. Am. Water Works Assoc. 1988, 80, 73–81. [Google Scholar] [CrossRef]
- Edwards, M.; Benjamin, M.M. A Mechanistic Study of Ozone-Induced Particle Destabilization. J. Am. Water Works Assoc. 1991, 83, 96–105. [Google Scholar] [CrossRef]
- Sadrnourmohamadi, M.; Gorczyca, B. Effects of ozone as a stand-alone and coagulation-aid treatment on the reduction of trihalomethanes precursors from high DOC and hardness water. Water Res. 2015, 73, 171–180. [Google Scholar] [CrossRef]
- Hoigné, J.; Bader, H. Rate constants of reactions of ozone with organic and inorganic compounds in water-I: non-dissociating organic compounds. Water Res. 1983, 17, 173–183. [Google Scholar] [CrossRef]
- Medeiros, D.R.; Pires, E.C.; Mohseni, M. Ozone oxidation of pulp and paper wastewater and its impact on molecular weight distribution of organic matter. Ozone Sci. Eng. 2008, 30, 105–110. [Google Scholar] [CrossRef]
- Ulucan-altuntas, K.; Ilhan, F. Enhancing biodegradability of textile wastewater by ozonation processes: Optimization with response surface methodology. Ozone Sci. Eng. 2018. [Google Scholar] [CrossRef]
- Gilbert, E. Biodegradability of ozonation products as a function of COD and DOC elimination by the example of humic acids. Water Res. 1988, 22, 123–126. [Google Scholar] [CrossRef]
- Yavich, A.A.; Lee, K.; Chen, K.; Pape, L.; Masten, S.J. Evaluation of biodegradability of NOM after ozonation. Water Res. 2004, 38, 2839–2846. [Google Scholar] [CrossRef] [PubMed]
- Von Gunten, U. Ozonation of drinking water: Part, I.I. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res. 2003, 37, 1469–1487. [Google Scholar] [CrossRef]
- Fang, J.; Liu, H.; Shang, C.; Zeng, M.; Ni, M.; Liu, W. E. coli and bacteriophage MS2 disinfection by UV; ozone and the combined UV and ozone processes. Front. Environ. Sci. Eng. 2014, 8, 547–552. [Google Scholar] [CrossRef]
- World Health Organization. Health Guidelines for the Use of Wastewater in Agriculture and Aquaculture; Technical Report Series No. 778; World Health Organization: Geneva, Switzerland, 1989. [Google Scholar]
- Martínez, S.B.; Pérez-parra, J.; Suay, R. Use of Ozone in Wastewater Treatment to Produce Water Suitable for Irrigation. Water Resour. Manag. 2011, 25, 2109–2124. [Google Scholar] [CrossRef]
- Elovitz, M.S.; Von, G.U. Hydroxyl Radical/Ozone Ratios During Ozonation Processes. I. The Rct Concept. Ozone Sci. Eng. 1999, 21, 239–260. [Google Scholar] [CrossRef]
- Papageorgiou, A.; Voutsa, D.; Papadakis, N. Occurrance and fate of ozonation by-products at a full-scale drinking water treatment plant. Sci. Total Environ. 2014, 481, 392–400. [Google Scholar] [CrossRef]
- Chon, K.; Salhi, E.; Von, G.U. Combination of UV absorbance and electron donating capacity to assess degradation of micropollutants and formation of bromate during ozonation of wastewater effluents. Water Res. 2015, 81, 388–397. [Google Scholar] [CrossRef]
- Klauson, D.; Klein, K.; Kivi, A.; Kattel, E.; Viisimaa, M.; Dulova, N.; Velling, S.; Trapido, M.; Tenno, T. Combined methods for the treatment of a typical hardwood soaking basin wastewater from plywood industry. Int. J. Environ. Sci. Technol. 2015, 12, 3575–3586. [Google Scholar] [CrossRef] [Green Version]
- Roth, J.A.; Sullivan, D.E. Kinetics of Ozone Decomposition in Water. Ozone Sci. Eng. 1983, 5, 37–49. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, L.; Hu, J. Investigation of ozonation kinetics and transformation products of sucralose. Sci. Total Environ. 2017, 603–604, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Crousier, C.; Jean-stéphane, P.; Albet, J.; Baig, S.; Roustan, M. Urban Wastewater Treatment by Catalytic Ozonation. Ozone Sci. Eng. 2015, 38, 3–13. [Google Scholar] [CrossRef]
- Marce, M.; Domenjoud, B.; Esplugas, S.; Baig, S. Ozonation treatment of urban primary and biotreated wastewaters: Impacts and modeling. Chem. Eng. J. 2016, 283, 768–777. [Google Scholar] [CrossRef]
- Beltrin, F.J.; Garcia-araya, J.F.; Álvarez, P.M. Domestic wastewater ozonation. Ozone Sci. Eng. 2007, 23, 219–228. [Google Scholar] [CrossRef]
- Shin, J.; Hidayat, Z.R.; Lee, Y.; Shin, J.; Hidayat, Z.R.; Lee, Y. Influence of Seasonal Variation of Water Temperature and Dissolved Organic Matter on Ozone and OH Radical Reaction Kinetics During Ozonation of a Lake Water. Ozone Sci. Eng. 2016, 38, 100–114. [Google Scholar] [CrossRef]
- Wen, G.; Ma, J.; Liu, Z.Q.; Zhao, L. Ozonation kinetics for the degradation of phthalate esters in water and the reduction of toxicity in the process of O3/H2O2. J. Hazard. Mater. 2011, 195, 371–377. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Priority pollutants. United States Environ. Prot. Agency. 2014. Available online: https://www.epa.gov/eg/toxic-and-priority-pollutants-under-clean-water-act (accessed on 9 February 2018).
- Sundell, J.; Bornehag, C.G. Presences of phthalate esters in homes. Epidemiology 2006, 17, S86. [Google Scholar] [CrossRef]
- Vitali, M.; Guidotti, M.; Macilenti, G.; Cremisini, C. Phthalate esters in freshwaters as markers of contamination sources—A site study in Italy. Environ. Int. 1997, 23, 337–347. [Google Scholar] [CrossRef]
- Mu, X.; Huang, Y.; Li, J.; Yang, K.; Yang, W.; Shen, G.; Li, X.; Lei, Y.; Pang, S.; Wang, C.; et al. New insights into the mechanism of phthalate-induced developmental effects. Environ. Pollut. 2018, 241, 674–683. [Google Scholar] [CrossRef]
- Morales-Garcia, S.S.; Rodriguez-Espinosa, P.F.; Shruti, V.C.; Jonathan, M.P.; Martinez-Tavera, E. Metal concentrations in aquatic environments of Puebla River basin, Mexico: Natural and industrial influences. Environ. Sci. Pollut. Res. 2016, 24, 2589–2604. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Espinosa, P.F.; Mendoza-Pérez, J.A.; Tabla-Hernandez, J.; Martínez-Tavera, E.; Monroy-Mendieta, M.M. Biodegradation and kinetics of organic compounds and heavy metals in an artificial wetland system (AWS) by using water hyacinths as a biological filter. Int J. Phytoremediation 2018, 20, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Shruti, V.C.; Jonathan, M.P.; Rodríguez-Espinosa, P.F.; Nagarajan, R.; Escobedo-Urías, D.C.; Morales-García, S.S.; et al. Geochemical characteristics of stream sediments from an urban-volcanic zone, Central Mexico: Natural and man-made inputs. Chemie der Erde-Geochemistry 2017, 77, 303–321. [Google Scholar] [CrossRef]
- Martinez-Tavera, E.; Rodriguez-Espinosa, P.F.; Shruti, V.C.; Sujitha, S.B.; Morales-Garcia, S.S.; Muñoz-Sevilla, N.P. Monitoring the seasonal dynamics of physicochemical parameters from Atoyac River basin (Puebla), Central Mexico: Multivariate approach. Environ. Earth Sci. 2017, 76, 95. [Google Scholar] [CrossRef]
- Rodriguez-Espinosa, P.F.; Morales-García, S.S.; Jonathan, M.P.; Navarrete-Lopez, M.; Bernal-Campos, A.A.; Gonzales-Cesar, A.; Muñoz-Sevilla, N.P. Servicio Ambiental de la Presa Valsequillo para las Cuencas de los Ríos del Atoyac-Zahuapan y Alseseca, Puebla, Tlaxcala, México. Inst Nac Ecol y Cambio Clim (INECC) Secr Medio Ambient y Recur Na. 2011, pp. 1–10. Available online: http://viveatoyac.org.mx/images/Biblioteca temática/Biblioteca sobre el agua/3g010 Servicio ambiental de la presa Valsequillo para las cuencas del Atoyac y Alseseca.pdf (accessed on 11 October 2018).
- Morales-García, S.S.; Rodriguez-Espinoza, P.F.; Jonathan, M.P. Environmental Assessment and Sustainable Development of Valsequillo in Puebla, Mexico. In Proceedings of the 2012 International Conference on Environmental Science and Technology, Houston, TX, USA, 25–29 June 2012; pp. 113–117. [Google Scholar]
- Kishimoto, N.; Morita, Y.; Tsuno, H.; Yasuda, Y. Characteristics of electrolysis, ozonation and their combination process on treatment of municipal wastewater. Water Environ. Fed. 2007, 79, 1033–1042. [Google Scholar] [CrossRef]
- Gárfias, J.; Arroyo, N.; Aravena, R. Hydrochemistry and origins of mineralized waters in the Puebla aquifer system, Mexico. Environ. Earth Sci. 2009, 59, 1789–1805. [Google Scholar] [CrossRef]
- Aldstadt, J.H., III; Bootsma, H.A.; Ammerman, J.L. Chemical Properies of Water. In Earth Systems and Environmental Sciences; Likens, G.E., Ed.; Springer: Basel, Switzerland, 2009. [Google Scholar]
- Tebbut, T.H.Y. Fundamentos de Control de la Calidad del agua; Editores ELSA de CVGN, Ed.; Editorial Limusa S.A. de C.V. Grupo Noriega Editors: Ciudad de México, Mexico, 1998. [Google Scholar]
- Boyd, C.E. Water Quality: An Introduction, 2nd ed.; Springer: Basel, Switzerland, 2008. [Google Scholar]
- Wert, E.C.; Rosario-ortiz, F.L.; Drury, D.D.; Snyder, S.A. Formation of oxidation byproducts from ozonation of wastewater. Water Res. 2007, 41, 1481–1490. [Google Scholar] [CrossRef]
- Martín-Loaiza, C.; Céspedes, C.L. Compuestos volátiles de plantas, origen, emisión, efectos, análisis y aplicaciones al agro. Rev. Fitotec. Mex. 2007, 30, 327–351. [Google Scholar]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk, J. The velocity of ozonation of benzene and its homologues. Recueil des Travaux Chimiques des Pays-Bas 1948, 67, 945–1019. [Google Scholar] [CrossRef]
- Hoigné, J.; Bader, H. Ozonation of water: “Oxidation-competition values” of different types of waters used in Switzerland. Ozone Sci. Eng. 1979, 1, 357–372. [Google Scholar] [CrossRef]
- Kwon, M.; Kye, H.; Jung, Y.; Yoon, Y.; Joon-wun, K. Performance characterization and kinetic modeling of ozonation using a new method: ROH,O3 concept. Water Res. 2017, 122, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Beltrin, F.J.; Garcia-Araya, J.F.; Álvarez, P.M. Domestic Wastewater Ozonation: A Kinetic Model Approach. Ozone Sci. Eng. 2001, 23, 219–228. [Google Scholar] [CrossRef]
- Papageorgiou, A.; Stylianou, S.K.; Kaffes, P.; Zouboulis, A.I.; Voutsa, D. Effects of ozonation pretreatment on natural organic matter and wastewater derived organic matter—Possible implications on the formation of ozonation by-products. Chemosphere 2017, 170, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Wert, E.C.; Rosario-ortiz, F.L.; Snyder, S.A. Effect of ozone exposure on the oxidation of trace organic contaminants in wastewater. Water Res. 2009, 43, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; Mckay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Hoigné, J. The Chemistry of Ozone in Water. In Process Technologies for Water Treatment, 1st ed.; Stucki, S., Ed.; Plenum Press: New York, NY, USA, 1988. [Google Scholar]
- Jekel, M.R. Flocculation Effects of Ozone. Ozone Sci. Eng. 1994, 16, 55–66. [Google Scholar] [CrossRef]
- Gutierrez-Lopez. Determinación de la Capacidad de Asimilación de Contaminantes en la presa Manuel Ávila Camacho, Puebla. Ph.D. Thesis, Instituto Mexicano de Tecnologia del Agua (IMTA), Jiutepec, Mexico, 2014. [Google Scholar]
- Reckhow, D.A.; Legube, B.; Singer, P.C. The ozonation of organic halide precursors: Effect of bicarbonate. Water Res. 1986, 20, 987–998. [Google Scholar] [CrossRef]
- Can, Z.S.; Gurol, M. Formaldehyde formation during ozonation of drinking water. Ozone Sci. Eng. 2003, 25, 41–51. [Google Scholar] [CrossRef]
- Reynolds, G.; Corless, C.; Graham, N.; Perry, R.; Gibson, T.M.; Haley, J. Aqueous Ozonation of Fatty Acids. Ozone Sci. Eng. 1989, 11, 143–154. [Google Scholar] [CrossRef]
- Kırış, S.; Velioglu, Y.S.; Tekin, A. Effect of Ozonated Water Treatment on Fatty Acid Composition and Some Quality Parameters of Olive Oil. Ozone Sci Eng. 2017, 39, 91–96. [Google Scholar] [CrossRef]
- Kianmehr, P.; Kfoury, F. Prediction of Methane Generation of Ozone-Treated Sludge from a Wastewater Treatment Plant. Ozone Sci. Eng. 2016, 38, 465–471. [Google Scholar] [CrossRef]
- Sevimli, M.F.; Sarikaya, H.Z.; Yazgan, M.S. A new approach to determine the practical ozone dose for color removal from textile wastewater. Ozone Sci. Eng. 2003, 25, 137–143. [Google Scholar] [CrossRef]
- Li, W.-X.; Tang, C.-D.; Wu, Z.-L.; Wang, W.-M.; Zhang, Y.-F.; Zhao, Y.; Cravotto, G. Eutrophic water purification efficiency using a combination of hydrodynamic cavitation and ozonation on a pilot scale. Environ. Sci. Pollut. Res. 2015, 22, 6298–6307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tian, Y.; Zhang, J. Release of phosphorus from sewage sludge during ozonation and removal by magnesium ammonium phosphate. Environ. Sci. Pollut. Res. 2017, 24, 23794–23802. [Google Scholar] [CrossRef] [PubMed]
- Guya, F.J. Bioavailability of particle-associated nutrients as affected by internal regeneration processes in the Nyanza Gulf region of Lake Victoria. Lakes Reserv. Res. Manag. 2013, 18, 129–143. [Google Scholar] [CrossRef]
- Giri, R.R.; Ozaki, H.; Ota, S.; Takanami, R.; Taniguchi, S. Degradation of common pharmaceuticals and personal care products in mixed solutions by advanced oxidation techniques. Int. J. Environ. Sci. Technol. 2010, 7, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Khamparia, S.; Jaspal, D.K. Adsorption in combination with ozonation for the treatment of textile waste water: A critical review. Front. Environ. Sci. Eng. 2017, 11, 1–18. [Google Scholar] [CrossRef]
- Fahmi, N.W.; Okada, M. Improvement of DOC removal by multi-stage AOP-biological treatment. Chemosphere 2003, 50, 1043–1048. [Google Scholar] [CrossRef]
- Von Gunten, U. Ozonation of drinking water: Part, I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- El-taliawy, H.; Ekblad, M.; Nilsson, F.; Hagman, M.; Paxeus, N.; Jönsson, K.; Cimbritz, M.; Jansen, J.C.; Bester, K. Ozonation efficiency in removing organic micro pollutants from wastewater with respect to hydraulic loading rates and different wastewaters. Chem. Eng. J. 2017, 325, 310–321. [Google Scholar] [CrossRef]
- Song, Y.; Breider, F.; Ma, J.; von Gunten, U. Nitrate formation during ozonation as a surrogate parameter for abatement of micropollutants and the N-nitrosodimethylamine (NDMA) formation potential. Water Res. 2017, 122, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Xie, S.; Ding, C.; Lin, F.; He, Y.; Wang, Z.; Cen, K. The Benefits of Small Quantities of Nitrogen in the Oxygen Feed to Ozone Generators. Ozone Sci. Eng. 2018, 40, 313–320. [Google Scholar] [CrossRef]
- Ruano, M.V.; Ribes, J.; Seco, A.; Ferrer, J. An advanced control strategy for biological nutrient removal in continuous systems based on pH and ORP sensors. Chem. Eng. J. 2012, 183, 212–221. [Google Scholar] [CrossRef]
- Yu, R.; Lin, C.; Chen, H.; Cheng, W.; Kao, M. Possible control approaches of the Electro-Fenton process for textile wastewater treatment using on-line monitoring of DO and ORP. Chem. Eng. J. 2013, 218, 341–349. [Google Scholar] [CrossRef]
- Goncharuk, V.V.; Bagrii, V.A.; Mel, L.A.; Chebotareva, R.D.; Bashtan, S.Y. The Use of Redox Potential in Water Treatment Processes. Phys. Chem. Water Treat. Process. 2010, 32, 1–9. [Google Scholar] [CrossRef]
- James, C.N.; Copeland, R.C.; Lytle, D.A. Relationships between oxidation-reduction potential, oxidant, and pH in drinking water. In Proceedings of the AWWA Water Quality Technology Conference, San Antonio, TX, USA, 14–18 November 2004. [Google Scholar]
- Klein, H.P. Ozone in Water Treatment Processes. In Process Technology Water Treatment, 1st ed.; Stucki, S., Ed.; Springer: Boston, MA, USA, 1988. [Google Scholar]
- Toui, S. The Oxidation of Manganese and Disinfection by Ozonation in Water Purification Processing. Ozone Sci. Eng. 1991, 13, 623–637. [Google Scholar] [CrossRef]
- Edwards, M.; Benjamin, M.M. Transformation of NOM by Ozone and its Effect on Iron and Aluminum Solunility. Res. Technol. 1992, 84, 56–66. [Google Scholar]
- Flores-Márquez, E.L.; Jiménez-Suárez, G.; Martínez-Serrano, R.G.; Chávez, R.E.; Pérez, D.S. Study of geothermal water intrusion due to groundwater exploitation in the Puebla Valley aquifer system, Mexico. Hydrogeol. J. 2006, 14, 1216–1230. [Google Scholar] [CrossRef]
- Rozan, T.F.; Benoit, G.; Luther, G.W. Measuring metal sulfide complexes in oxic river waters with square wave voltammetry. Environ. Sci. Technol. 1999, 33, 3021–3026. [Google Scholar] [CrossRef]
- Buffle, J. Natural Organic Matter and Metal-Organic interaction in Aquatic Systems. In Metal Ions in Biological Systems; Sigel, H., Ed.; University of Basel: Basel, Switzerland, 1984; p. 399. [Google Scholar]
- Zeng, F.; Cui, K.; Xie, Z.; Liu, M.; Li, Y.; Lin, Y.; Zeng, Z.; Li, F. Occurrence of phthalate esters in water and sediment of urban lakes in a subtropical city, Guangzhou, South China. Environ. Int. 2008, 34, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.; Tosine, H.; Onuska, F.I.; Comba, M.E. The ozonation of natural waters: Product identification. Ozone Sci. Eng. 1980, 2, 55–64. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A.; Madhavan, A.; Alphonsa, J.A.; Vivek, N.; Gnansounou, E.; Castro, E.; Faraco, V. Water hyacinth a potential source for value addition: An overview. Bioresour. Technol. 2017, 230, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.P. Ozone pretreatment to increase digestibility of lignocellulose. Biotechnol. Lett. Vol. 1983, 5, 773–776. [Google Scholar] [CrossRef]
- Binder, A.; Pelloni, L.; Fiechter, A. Delignification of Straw with Ozone to Enhance Biodegradability. Appl. Microbiol. Biotechnol. 1980, 11, 1–5. [Google Scholar] [CrossRef]
- Zappi, M.E.; Hernandez, R.; Gang, D.; Bajpai, R.; Kuo, C.H.; Hill, D.O. Treatment of groundwater contaminated with high levels of explosives using advanced oxidation processes. Int. J. Environ. Sci. Technol. 2016, 13, 2767–2778. [Google Scholar] [CrossRef]
- Rodríguez, A.; Rosal, R.; Perdigón-Melón, J.A.; Mezcua, M.; Agüera, A.; Hernando, M.D.; Letón, P.; Fernández-Alba, A.R.; García-Calvo, E. Ozone-Based Technologies in Water and Wastewater Treatment. Handb. Environ. Chem. 2008, 5, 127–175. [Google Scholar]
- NOM-001-SEMARNAT-1996. Límites Máximos Permisibles De Contaminantes En Las Descargas De Aguas Residuales En Aguas Y Bienes Nacionales. Secr. medio Ambient. y Recur. Nat. D. Of. la Fed. 1996. Available online: http://www.gob.mx/cms/uploads/attachment/file/105139/Normas_Oficiales_Mexicanas.pdf (accessed on 21 November 2018).







| (a) Parameters Measured in CIIEMAD-IPN | ||||
| Parameter | Units | Equipment Employed | Local Method | International Concordance |
| Chemical oxygen demand (COD) | mg/L | Spectrophotometry UV/Vis (PerkinElmerLambda 20) | NMX-AA-030/1-SCFI-2012 | (APHA et al., 2005)/5220D |
| Biochemical oxygen demand (BOD) | mg/L | Oximeter (YSI/51B) | NMX-AA-028-SCFI-2001 | (APHA et al., 2005)/5210B |
| Spectral absorption coefficient at 254 nanometers (SAC254) | Abs/m | Spectrophotometry UV/Vis (PerkinElmerLambda 20) with 1 cm cell (q) | - | - |
| Turbidity | NTU | Turbidimeter (model 2100Q) | NOM-AA-38-SCFI-1981 | (APHA et al., 2005)/2130B |
| Hydrogen potential (pH) | - | Multip. HACH (Model 2100Q & HQ40D) | NMX-AA-008-SCFI-2011 | (APHA et al., 2005)/4500B |
| Conductivity | µS/cm | Multip. HACH (Model 2100Q & HQ40D) | NMX-AA-093-SCFI-2000 | (APHA et al., 2005)/2510B |
| Dissolved oxygen (DO) | mg/L | Multip. HACH (Model 2100Q & HQ40D) | NMX-AA-012-SCFI-2001 | (APHA et al., 2005)/2810 |
| Oxidation-Reduction potential | mV | Multip. HACH (Model 2100Q & HQ40D) | - | (APHA et al., 2005)/2580 |
| Fecal coliforms (FC) | NMP/100 mL | - | NMX-AA-042-SCFI-2015 | (APHA et al., 2005)/9221 |
| Alkalinity (Alky) | mg CaCO3/L | - | NMX-AA-036-SCFI-2001 | (APHA, 2012)/2320B |
| (b) Parameters Measured in CMPL-IPN | ||||
| Parameter | Units | Equipment Employed | Local Method | International Concordance |
| Total solids | mg/L | Stove (Model Riossa Series E-33) | NMX-AA-034-SCFI-2001 | (APHA et al., 2005)/2540B |
| Total dissolved solids | mg/L | Stove (Model Riossa Series E-33) | NMX-AA-034-SCFI-2001 | (APHA et al., 2005)/2540C |
| Total suspended solids | mg/L | Stove (Model Riossa Series E-33) | NMX-AA-034-SCFI-2001 | (APHA et al., 2005)/2540D |
| (c) Parameters Measured in Intertek + ABC—Analytic Laboratories | ||||
| Parameter | Units | Equipment Employed | Local Method | International Concordance |
| Real color (RC) | Pt-Co | Aqua tester, Orbeco/Hellige (Model C611A) | NMX-AA-045-SCFI-2001 | - |
| Fats and Oils (FaO) | mg/L | Extraction by using hexane as solvent | NMX-AA-005-SCFI- 2013 | - |
| Total phosphorous (TP) | (mg/L) | FA′S OI Analytical—Flow Solution IV | NMX-AA-029-SCFI-2001 | - |
| Ammonia nitrogen (NH3-N) | mg/L | FA′S OI Analytical—Flow Solution IV | NMX-AA-026-SCFI-2010 | US EPA 350.1-1993 (I) |
| Nitrates NO3− | mg/L | FA′S OI Analytical—Flow Solution IV | NMX-AA-079-SCFI-2001 | US EPA 353.2-1993 (I) |
| Sulfates (SO42−) | mg/L | FA′S OI Analytical—Flow Solution IV | - | US EPA 9036-1986 |
| Chloride (Cl−) | mg/L | FA′S OI Analytical—Flow Solution IV | NMX-AA-073-SCFI-2001 | - |
| Total Cyanides (CN−) | mg/L | FA′S OI Analytical—Flow Solution IV | NMX-AA-058-SCFI-2001 | US EPA 335.3-1978 (I) |
| Al, Cr, Cu, Fe, Zn, Mn and Ni | µg/L | ICP-OES CID Thermo Scienctific-6500 | NMX-AA-051-SCFI-2001 | US EPA 6010C 2007 (I) |
| Hg | µg/L | Mercury analyzer Hydra IIAA | NMX-AA-051-SCFI-2001 | US EPA 7470A 1994 (I) |
| Dimethyl phthalate (DMP), Diethyl phthalate (DEP), Dibutyl phthalate (DBP), Di(2-ethylhexyl) phthalate (DEHP) and Di(n-octil)ftalato (DINP) | µg/L | Gas chromatography (GC/MSD) Agilent technologies 7890B-5977 A MSD | - | US EPA 8270D 2007 |
| Isophorone (IP) | µg/L | Gas chromatography (GC/MSD) Agilent technologies 7894 B/5977 A MSD | - | US EPA 8270D 2007 |
| Total Phenols (TPh) | µg/L | FA′S OI Analytical—Flow Solution IV | - | US EPA 8270D 2007 |
| Water Sample | Time (m) | Ozone Dose * | Turbidity (NTU) | Alky a | FaO b (mg/L) | TP c (mg/L) | pH | σ (µS/cm) | NH3-N (mg/L) | NO3− (mg/L) | DO e (mg/L) | ORP f (mV) | RC g (Pt-Co) | SO42− (mg/L) | Cl− (mg/L) | CN− (mg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) VO3-AT | 0 | 0.0 | 182 | 277 | 6.0 | 3.6 | 7.4 | 1153 | 13.9 | 0.04 | 4.2 | 110 | 30 | 85.7 | 56.0 | 4.2 |
| 5 | 0.2 | 182 | - | - | - | 7.9 | 1137 | - | - | 7.4 | 119 | - | - | - | - | |
| 20 | 0.8 | 169 | - | - | - | 8.0 | 1148 | - | - | 7.0 | 119 | - | - | - | - | |
| 60 | 2.3 | 145 | - | - | - | 8.4 | 1177 | - | - | 6.5 | 115 | - | - | - | - | |
| 120 | 4.7 | 93 | - | 6.7 | 1.6 | 8.5 | 1170 | 13.4 | 0.35 | 6.8 | 113 | 15 | 78.4 | 57.0 | 1.7 | |
| (b) VO3-AL | 0 | 0.0 | 182 | 383 | - | - | 7.6 | 827 | - | - | 5.3 | 123 | - | - | - | - |
| 5 | 0.7 | 164 | - | - | - | 8.1 | 841 | - | - | 7.0 | 126 | - | - | - | - | |
| 20 | 0.3 | 140 | - | - | - | 8.4 | 846 | - | - | 7.0 | 126 | - | - | - | - | |
| 60 | 0.8 | 132 | - | - | - | 8.5 | 854 | - | - | 6.7 | 129 | - | - | - | - | |
| 120 | 1.6 | 114 | - | - | - | 8.6 | 848 | - | - | 6.5 | 120 | - | - | - | - | |
| (c) VO3-01 | 0 | 0.0 | 58 | 235 | 7.2 | 2.0 | 7.3 | 643 | 10.1 | 0.01 | 3.6 | 137 | 30 | 66.4 | 45.0 | 3.3 |
| 5 | 0.5 | 50 | - | - | - | 7.9 | 646 | - | - | 7.3 | 132 | - | - | - | - | |
| 20 | 2.1 | 52 | - | - | - | 8.2 | 648 | - | - | 7.4 | 131 | - | - | - | - | |
| 60 | 6.3 | 45 | - | 9.6 | 1.3 | 8.4 | 650 | 10.0 | 0.25 | 7.1 | 125 | 15 | 69.8 | 48.0 | 1.8 | |
| (d) VO3-02 | 0 | 0.0 | 8 | 263 | ND | 1.9 | 7.5 | 681 | 9.8 | 0.02 | 7.2 | 127 | 50 | 62.9 | 60.0 | - |
| 5 | 3.3 | 7 | - | - | - | 7.6 | 734 | - | - | 6.9 | 134 | - | - | - | - | |
| 15 | 10.0 | 9 | - | - | - | 8.3 | 723 | - | - | 7.7 | 130 | - | - | - | - | |
| 40 | 26.7 | 9 | - | ND | 2.0 | 8.4 | 745 | 10.3 | 0.12 | 6.7 | 115 | 40 | 58.9 | 59.0 | - | |
| (e) VO3-03 | 0 | 0.0 | 12 | 267 | - | - | 7.2 | 714 | - | - | 6.2 | 134 | - | - | - | - |
| 5 | 3.3 | 11 | - | - | - | 7.3 | 738 | - | - | 6.8 | 150 | - | - | - | - | |
| 15 | 10.0 | 11 | - | - | - | 8.0 | 746 | - | - | 6.9 | 145 | - | - | - | - | |
| 30 | 20.0 | 11 | - | - | - | 8.4 | 756 | - | - | 6.8 | 135 | - | - | - | - |
| Time (m) | O3 (mg) | (a) VO3-AL | (b) VO3-AT | (c) VO3-01 | (d) VO3-02 | (e) VO3-03 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COD a | BOD5 b | SAC254 c | COD a | BOD5 b | SAC254 c | COD a | BOD5 b | SAC254 c | COD a | BOD5 b | SAC254 c | COD a | BOD5 b | SAC254 c | ||
| 0 | 0.0 | 505.0 | 181.5 | 64 | 172.0 | 59.5 | 113 | 64.0 | 22.5 | 43 | 10.0 | 9.6 | 30 | 10.0 | 10.0 | 30 |
| 5 | 33.3 | 371.0 | 156.9 | 63 | 178.0 | 42.0 | 112 | 39.0 | - | 42 | 19.7 | 9.8 | 28 | 10.0 | 10.0 | 27 |
| 15 | 100.0 | - | - | - | - | - | - | - | 10.0 | 8.1 | 27 | 10.0 | 10.0 | 26 | ||
| 20 | 133.3 | 201.0 | 117.8 | 56 | 133.0 | 36.6 | 110 | 25.0 | 14.8 | 36 | - | - | - | - | - | - |
| 30 | 200.0 | - | - | - | - | - | - | - | - | - | - | 10.0 | 10.0 | 24 | ||
| 40 | 267.0 | - | - | - | - | - | - | - | 10.0 | 10.0 | 20 | - | - | - | ||
| 60 | 400.0 | 294.0 | 109.6 | 56 | 130.0 | 62.5 | 110 | 32.0 | 12.6 | 36 | - | - | - | - | - | - |
| 120 | 800.0 | 305.0 | 91.6 | 54 | 88.0 | 26.8 | 101 | - | - | - | - | - | - | - | - | - |
| Water Sample | Zero Order | First Order | Second Order a | |||
|---|---|---|---|---|---|---|
| K b | (R2) c | K b | (R2) c | Kb | (R2) c | |
| (a) COD d | ||||||
| VO3-AL | 14.3290 | 0.9556 | 0.0450 | 0.9910 | 0.0002 | 0.9998 |
| VO3-AT | 0.6807 | 0.8751 | 0.0054 | 0.9139 | 0.0004 | 0.9289 |
| VO3-01 | 1.6921 | 0.8112 | 0.0426 | 0.9000 | 0.0012 | 0.9651 |
| VO3-02 | - | - | - | - | - | - |
| VO3-03 | - | - | - | - | - | - |
| (b) BOD5 e | ||||||
| VO3-AL | 0.6229 | 0.7200 | 0.0049 | 0.7913 | 0.0004 | 0.8573 |
| VO3-AT | 0.9658 | 0.7018 | 0.0208 | 0.7472 | 0.0005 | 0.7942 |
| VO3-01 | 0.1492 | 0.7675 | 0.0088 | 0.8147 | 0.0005 | 0.8627 |
| VO3-02 | - | - | - | - | - | - |
| VO3-03 | - | - | - | - | - | - |
| (c) SAC f | ||||||
| VO3-AL | 0.0931 | 0.8904 | 0.0090 | 0.8924 | 8 × 10−6 | 0.8899 |
| VO3-AT | 0.0774 | 0.6850 | 0.0013 | 0.6998 | 2 × 10−5 | 0.7147 |
| VO3-01 | 0.3274 | 0.9979 | 0.0082 | 0.9965 | 0.0002 | 0.9948 |
| VO3-02 | 0.2357 | 0.9916 | 0.0093 | 0.9875 | 0.0004 | 0.9803 |
| VO3-03 | 0.1764 | 0.9375 | 0.0066 | 0.9494 | 0.0002 | 0.9586 |
| Total Metals (µg/L) | Organic Compounds (µg/L) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water Sample | Ozone Dose * | Al | Cr | Cu | Fe | Zn | Mn | Ni | Hg | DEHP a | DBP b | DEP c | DMP d | DINP e | IP f | TPh g |
| VO3-AT | Before (0) | 6790.0 | 13.4 | 29.1 | 7008.5 | 110.3 | 576.0 | 24.1 | 0.2 | 5.8 | ND | ND | ND | ND | 0.5 | 53.0 |
| After (4.7) | 660.0 | ND | 5.8 | 684.0 | 20.5 | 154.0 | 7.9 | 0.0 | 4.7 | 2.9 | ND | ND | 0.9 | 0.4 | 43.7 | |
| VO3-01 | Before (0) | 2250.0 | 5.4 | 8.8 | 2275.6 | 40.4 | 365.0 | 11.3 | 0.0 | ND | ND | ND | ND | ND | ND | 47.9 |
| After (6.3) | 450.0 | ND | 51.8 | 697.5 | 48.2 | 230.0 | 181.2 | 0.0 | 5.6 | 6.9 | 0.4 | 0.6 | ND | 0.1 | 48.3 | |
| VO3-02 | Before (0) | 60.0 | ND | ND | 138.7 | 15.6 | 293.0 | 6.2 | ND | ND | ND | ND | ND | ND | ND | 54.8 |
| After (26.7) | 70.0 | ND | ND | 197.1 | 12.4 | 318.0 | 7.5 | 0.1 | 1.6 | ND | ND | ND | ND | ND | 57.0 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabla-Hernández, J.; Rodríguez-Espinosa, P.F.; Hernandez-Ramirez, A.G.; Mendoza-Pérez, J.A.; Cano-Aznar, E.R.; Martínez-Tavera, E. Treatment of Eutrophic Water and Wastewater from Valsequillo Reservoir, Puebla, Mexico by Means of Ozonation: A Multiparameter Approach. Water 2018, 10, 1790. https://doi.org/10.3390/w10121790
Tabla-Hernández J, Rodríguez-Espinosa PF, Hernandez-Ramirez AG, Mendoza-Pérez JA, Cano-Aznar ER, Martínez-Tavera E. Treatment of Eutrophic Water and Wastewater from Valsequillo Reservoir, Puebla, Mexico by Means of Ozonation: A Multiparameter Approach. Water. 2018; 10(12):1790. https://doi.org/10.3390/w10121790
Chicago/Turabian StyleTabla-Hernández, J., P. F. Rodríguez-Espinosa, A. G. Hernandez-Ramirez, Jorge A. Mendoza-Pérez, E. R. Cano-Aznar, and E. Martínez-Tavera. 2018. "Treatment of Eutrophic Water and Wastewater from Valsequillo Reservoir, Puebla, Mexico by Means of Ozonation: A Multiparameter Approach" Water 10, no. 12: 1790. https://doi.org/10.3390/w10121790