An Experimental and Theoretical Study on Separations by Vacuum Membrane Distillation Employing Hollow-Fiber Modules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membrane Module
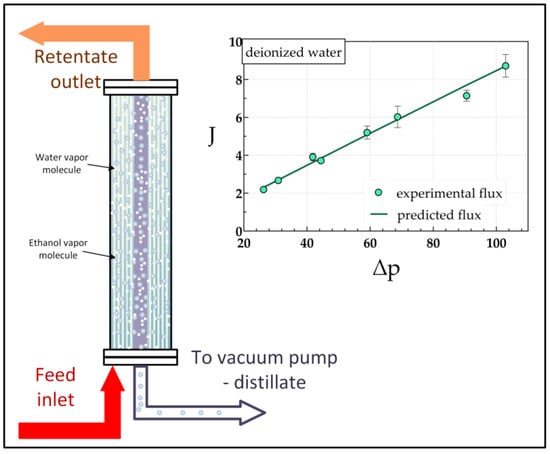
2.2. Experimental Set-Up and Experimental Protocol
2.3. Types of Feed-Solutions and Experimental Conditions
2.4. Cleaning Procedure
2.5. Analytical Methods
3. Results
3.1. Deionized Water
3.2. Desalination
3.3. Synthetic Ethanol-Water Solutions
3.4. Fermented Broth Solutions
3.5. Distillate of the Fermented Broth
4. Theoretical Analysis and Discussion
- In all performed experiments, the reduction of the liquid mass along the flow was less than 1%, thus, it can be safely ignored for the purpose of experimental data analysis.
- In all experiments, the temperature difference between inlet and outlet flows never exceeded 5 °C. This means that the complete problem can be linearized around the average temperature along the flow. The linearization is certainly accurate for the required interval of only 2.5 °C (in the worst case). Then the temperature can be assumed to be uniform along the flow and equal to its average, which is calculated as the mean value of measured inlet and outlet temperatures. This approach is very accurate since the error is proportional to the second order of the Taylor expansion of the vapor pressure–temperature function which is very small for small values of ΔT.
- Regarding temperature polarization, the temperature difference between the cup-mixing temperature and the membrane surface temperature is given by JΔH/ht [45], where J is the evaporation flux, ΔH the evaporation enthalpy, and ht the heat transfer coefficient. It is noted that, in cases of more than one evaporating species, the numerator must be replaced by a sum over the species. A simple computation shows that this difference is of the order of 0.1 °C, thus, it can be ignored since the experimental error is certainly larger.
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Membrane Chemical Composition | PP |
| membrane physical properties | |
| thickness | 450 μm ± 50 μm |
| inside diameter, din | 1800 μm ± 150 μm |
| nominal pore diameter, dp | 0.22 μm |
| maximum pore diameter | 0.6 μm |
| membrane porosity, εm | 73% |
| membrane performance characteristics | |
| bubble point (isopropyl alcohol, 23 °C) | 0.95 bar |
| transmembrane flow (isopropyl alcohol, 23 °C) | ≥2.1 mL/(min cm2 bar) |
| bacterial retension (brevundimonas diminuta) | ≥7 log reduction value |
| module characteristics | |
| M1 | Μ2 |
| mode of operation: inside out | Inside out |
| shell diameter: 40 mm | 40 mm |
| number of fibers: Μ1Α: 44, Μ1Β: 34 | 40 |
| fiber length: 350 mm | 350 mm |
| effective area (din): Μ1Α: 0.087 m2, M1B: 0.067 m2 | 0.079 m2 |
Appendix B


References
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T. MD membrane modules. In Membrane Distillation; Elsevier: Amsterdam, The Netherlands, 2011; pp. 227–247, Chapter 9. [Google Scholar]
- Khayet, M.; Matsuura, T. Vacuum membrane distillation. In Membrane Distillation; Elsevier: Amsterdam, The Netherlands, 2011; pp. 323–359, Chapter 12. [Google Scholar]
- Abu-Zeid, M.A.E.-R.; Zhang, Y.; Dong, H.; Zhang, L.; Chen, H.-L.; Hou, L. A comprehensive review of vacuum membrane distillation technique. Desalination 2015, 356, 1–14. [Google Scholar] [CrossRef]
- Susanto, H. Towards practical implementations of membrane distillation. Chem. Eng. Process. Process Intensif. 2011, 50, 139–150. [Google Scholar] [CrossRef]
- Chiam, C.K.; Sarbatly, R. Vacuum membrane distillation processes for aqueous solution treatment—A review. Chem. Eng. Process. Process Intensif. 2013, 74, 27–54. [Google Scholar] [CrossRef]
- Singh, D.; Li, L.; Obusckovic, G.; Chau, J.; Sirkar, K.K. Novel cylindrical cross-flow hollow fiber membrane module for direct contact membrane distillation-based desalination. J. Membr. Sci. 2018, 545, 312–322. [Google Scholar] [CrossRef]
- Singh, D.; Sirkar, K.K. Performance of PVDF flat membranes and hollow fibers in desalination by direct contact membrane distillation at high temperatures. Sep. Purif. Technol. 2017, 187, 264–273. [Google Scholar] [CrossRef]
- González, D.; Amigo, J.; Suárez, F. Membrane distillation: Perspectives for sustainable and improved desalination. Renew. Sustain. Energy Rev. 2017, 80, 238–259. [Google Scholar] [CrossRef]
- Naidu, G.; Choi, Y.; Jeong, S.; Hwang, T.M.; Vigneswaran, S. Experiments and modeling of a vacuum membrane distillation for high saline water. J. Ind. Eng. Chem. 2014, 20, 2174–2183. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, M.; Sun, D.; Li, B.; Li, P. Evaluation of commercial PTFE membranes for desalination of brine water through vacuum membrane distillation. Chem. Eng. Process. Process Intensif. 2016, 110, 52–63. [Google Scholar] [CrossRef]
- Mericq, J.P.; Laborie, S.; Cabassud, C. Vacuum membrane distillation of seawater reverse osmosis brines. Water Res. 2010, 44, 5260–5273. [Google Scholar] [CrossRef] [PubMed]
- Rom, A.; Strommer, M.; Friedl, A. Comparison of sweepgas and vacuum membrane distillation as in-situ separation of ethanol from aqueous solutions. Chem. Eng. Trans. 2014, 39, 985–990. [Google Scholar]
- Shi, J.Y.; Zhao, Z.P.; Zhu, C.Y. Studies on simulation and experiments of ethanol–water mixture separation by VMD using a PTFE flat membrane module. Sep. Purif. Technol. 2014, 123, 53–63. [Google Scholar] [CrossRef]
- Sarti, G.C.; Gostoli, C.; Bandini, S. Extraction of organic components from aqueous streams by vacuum membrane distillation. J. Membr. Sci. 1993, 80, 21–33. [Google Scholar] [CrossRef]
- Kujawski, W.; Kujawa, J.; Wierzbowska, E.; Cerneaux, S.; Bryjak, M.; Kujawski, J. Influence of hydrophobization conditions and ceramic membranes pore size on their properties in vacuum membrane distillation of water–organic solvent mixtures. J. Membr. Sci. 2016, 499, 442–451. [Google Scholar] [CrossRef]
- Urtiaga, A.M.; Ruiz, G.; Ortiz, I. Kinetic analysis of the vacuum membrane distillation of chloroform from aqueous solutions. J. Membr. Sci. 2000, 165, 99–110. [Google Scholar] [CrossRef]
- Bagger-Jørgensen, R.; Meyer, A.S.; Pinelo, M.; Varming, C.; Jonsson, G. Recovery of volatile fruit juice aroma compounds by membrane technology: Sweeping gas versus vacuum membrane distillation. Innov. Food Sci. Emerg. Technol. 2011, 12, 388–397. [Google Scholar] [CrossRef]
- Hasanoğlu, A.; Rebolledo, F.; Plaza, A.; Torres, A.; Romero, J. Effect of the operating variables on the extraction and recovery of aroma compounds in an osmotic distillation process coupled to a vacuum membrane distillation system. J. Food Eng. 2012, 111, 632–641. [Google Scholar] [CrossRef]
- Zhao, Z.P.; Ma, F.W.; Liu, W.-F.; Liu, D.Z. Concentration of ginseng extracts aqueous solution by vacuum membrane distillation. 1. Effects of operating conditions. Desalination 2008, 234, 152–157. [Google Scholar] [CrossRef]
- Zhao, Z.P.; Zhu, C.Y.; Liu, D.Z.; Liu, W.F. Concentration of ginseng extracts aqueous solution by vacuum membrane distillation 2. Theory analysis of critical operating conditions and experimental confirmation. Desalination 2011, 267, 147–153. [Google Scholar] [CrossRef]
- Jia, F.; Wang, J. Separation of cesium ions from aqueous solution by vacuum membrane distillation process. Prog. Nucl. Energy 2017, 98, 293–300. [Google Scholar] [CrossRef]
- Jia, F.; Li, J.; Wang, J.; Sun, Y. Removal of strontium ions from simulated radioactive wastewater by vacuum membrane distillation. Ann. Nucl. Energy 2017, 103, 363–368. [Google Scholar] [CrossRef]
- Plattner, J.; Naidu, G.; Wintgens, T.; Vigneswaran, S.; Kazner, C. Fluoride removal from groundwater using direct contact membrane distillation (DCMD) and vacuum enhanced DCMD (VEDCMD). Sep. Purif. Technol. 2017, 180, 125–132. [Google Scholar] [CrossRef]
- Dao, T.D.; Laborie, S.; Cabassud, C. Direct As(iii) removal from brackish groundwater by vacuum membrane distillation: Effect of organic matter and salts on membrane fouling. Sep. Purif. Technol. 2016, 157, 35–44. [Google Scholar] [CrossRef]
- Criscuoli, A.; Bafaro, P.; Drioli, E. Vacuum membrane distillation for purifying waters containing arsenic. Desalination 2013, 323, 17–21. [Google Scholar] [CrossRef]
- Peydayesh, M.; Kazemi, P.; Bandegi, A.; Mohammadi, T.; Bakhtiari, O. Treatment of bentazon herbicide solutions by vacuum membrane distillation. J. Water Process Eng. 2015, 8, e17–e22. [Google Scholar] [CrossRef]
- Carnevale, M.C.; Gnisci, E.; Hilal, J.; Criscuoli, A. Direct contact and vacuum membrane distillation application for the olive mill wastewater treatment. Sep. Purif. Technol. 2016, 169, 121–127. [Google Scholar] [CrossRef]
- Sivakumar, M.; Ramezanianpour, M.; O’Halloran, G. Mine water treatment using a vacuum membrane distillation system. APCBEE Procedia 2013, 5, 157–162. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Z.; Zhang, C.; Luan, J. Exploration and optimization of two-stage vacuum membrane distillation process for the treatment of saline wastewater produced by natural gas exploitation. Desalination 2016, 385, 117–125. [Google Scholar] [CrossRef]
- Criscuoli, A.; Zhong, J.; Figoli, A.; Carnevale, M.C.; Huang, R.; Drioli, E. Treatment of dye solutions by vacuum membrane distillation. Water Res. 2008, 42, 5031–5037. [Google Scholar] [CrossRef] [PubMed]
- Gryta, M.; Markowska-Szczupak, A.; Bastrzyk, J.; Tomczak, W. The study of membrane distillation used for separation of fermenting glycerol solutions. J. Membr. Sci. 2013, 431, 1–8. [Google Scholar] [CrossRef]
- Tomaszewska, M.; Białończyk, L. Production of ethanol from lactose in a bioreactor integrated with membrane distillation. Desalination 2013, 323, 114–119. [Google Scholar] [CrossRef]
- Gryta, M.; Barancewicz, M. Separation of volatile compounds from fermentation broth by membrane distillation. Pol. J. Chem. Technol. 2011, 13, 56–60. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewska, M.; Białończyk, L. The investigation of ethanol separation by the membrane distillation process. Pol. J. Chem. Technol. 2011, 13, 66–69. [Google Scholar] [CrossRef] [Green Version]
- Lewandowicz, G.; Białas, W.; Marczewski, B.; Szymanowska, D. Application of membrane distillation for ethanol recovery during fuel ethanol production. J. Membr. Sci. 2011, 375, 212–219. [Google Scholar] [CrossRef]
- Gryta, M. The fermentation process integrated with membrane distillation. Sep. Purif. Technol. 2001, 24, 283–296. [Google Scholar] [CrossRef]
- Gryta, M.; Morawski, A.W.; Tomaszewska, M. Ethanol production in membrane distillation bioreactor. Catal. Today 2000, 56, 159–165. [Google Scholar] [CrossRef]
- Gryta, M. Influence of polypropylene membrane surface porosity on the performance of membrane distillation process. J. Membr. Sci. 2007, 287, 67–78. [Google Scholar] [CrossRef]
- Barancewicz, M.; Gryta, M. Ethanol production in a bioreactor with an integrated membrane distillation module. Chem. Pap. 2012, 66, 85–91. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1990. [Google Scholar]
- Smolders, K.; Franken, A.C.M. Terminology for membrane distillation. Desalination 1989, 72, 249–262. [Google Scholar] [CrossRef]
- Poling, B.E.; Thompson, G.H.; Friend, D.G.; Rowley, R.L.; Wilding, W.V. Vapor pressures of pure substances. In Perry’s Chemical Engineers’ Handbook, 8th ed.; Green, D.W., Perry, R.H., Eds.; McGraw Hill Professional, Access Engineering: New York, NY, USA, 2008. [Google Scholar]
- Bandini, S.; Sarti, G.C. Heat and mass transport resistances in vacuum membrane distillation per drop. AIChE J. 1999, 45, 1422–1433. [Google Scholar] [CrossRef]









| Feed Solution | Average Concentration | Type of Module | Cross Flow Velocity | Temperature | Vacuum, Mbar | Mode |
|---|---|---|---|---|---|---|
| Deionized water | - | M2 | 0.2 m/s | 34.4–58.1 °C | 28–50 | Continuous |
| Synthetic brackish water | 3000 mg/L NaCl eC = 5733 ± 155 μS/cm | M1A M1B | 0.2 m/s | 42.3–65.7 °C | 60–130 | Continuous |
| Ethanol-water mixtures | 5.2 ± 0.5% v/v 12.3 ± 1.6% v/v | M1B M1A | 0.2 m/s | 26.7–47.0 °C 30.4–40.7 °C | 62–114 | Continuous |
| Fermented broth + UF 1 | 5.4 ± 0.5% v/v | M1B | 0.2 m/s | 29.3–38.2 °C | - | Semi-continuous |
| Fermented broth + UF | 6.5 ± 0.5% v/v | M1B | 0.2 m/s | 33.0 °C | - | Batch |
| Fermented broth + UF + NF 2 | 5.1 ± 0.5% v/v | M1B | 0.2 m/s | 36.1 °C | - | Batch |
| Fermented broth + UF | 12.5 ± 1% v/v | Μ1Α | 0.2 m/s | 34.0 °C | 65–122 | Semi-continuous |
| Fermented broth + UF + NF | 10.5 ± 1% v/v | Μ1Α | 0.2 m/s | 31.0 °C | - | Semi-continuous |
| Distillate | 14.0 ± 1% v/v | Μ1Α | 0.1 m/s | 32.0 °C | - | Semi-continuous |
| Average Feed Concentration, % v/v | Average Temperature Range, °C | Average Distillate Concentration, % v/v | Selectivity | Concentration Factor | Flux, kg/m2 h |
|---|---|---|---|---|---|
| 5.2 | 26.7−47.0 | 29.3 ± 3.3 | 7.4 ± 1.0 | 5.6 ± 0.6 | 0.6−1.7 |
| 12.3 | 30.4−40.7 | 49.4 ± 3.8 | 6.7 ± 0.7 | 4.1 ± 0.4 | 1.0−3.2 |
| Feed Solution | Mode | Feed Concentration, % v/v | Temperature, °C | Flux, kg/m2 h | Concentration Factor | Selectivity |
|---|---|---|---|---|---|---|
| Broth Fermented in MBR 1 | Semi-continuous | 5.4 | 29.3–38.2 | 1.2–2.1 | 2.9–4.2 | 5.3–7.7 |
| Broth Fermented in MBR | Batch | 6.5 | 33.0 ± 0.5 | 1.7 | 2.6 | 5.5 |
| Broth Fermented in MBR + NF | Batch | 5.1 | 36.5 ± 3.5 | 1.3 | 3.4 | 4.2 |
| Fermented Broth + UF | Semi-continuous | 12.5 | 34.0 ± 0.3 | 1.9 | 3.4 | 6.7 |
| Fermented Broth + UF + NF | Semi-continuous | 10.5 | 31 | 0.5 | 3.8 | 8 |
| Feed Solution | Mode | Feed Concentration, % v/v | Temperature, °C | Flux, kg/m2 h | Concentration Factor | Selectivity |
|---|---|---|---|---|---|---|
| Distillate | Semi-continuous | 14 | 33 | 1.7 | 3.4 | 6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karanasiou, A.; Kostoglou, M.; Karabelas, A. An Experimental and Theoretical Study on Separations by Vacuum Membrane Distillation Employing Hollow-Fiber Modules. Water 2018, 10, 947. https://doi.org/10.3390/w10070947
Karanasiou A, Kostoglou M, Karabelas A. An Experimental and Theoretical Study on Separations by Vacuum Membrane Distillation Employing Hollow-Fiber Modules. Water. 2018; 10(7):947. https://doi.org/10.3390/w10070947
Chicago/Turabian StyleKaranasiou, Anthoula, Margaritis Kostoglou, and Anastasios Karabelas. 2018. "An Experimental and Theoretical Study on Separations by Vacuum Membrane Distillation Employing Hollow-Fiber Modules" Water 10, no. 7: 947. https://doi.org/10.3390/w10070947